Final ID: 4370942
Deep Learning–Based Continuous QT Monitoring Identifies High-Risk Prolongation Events After Class III Antiarrhythmic Initiation
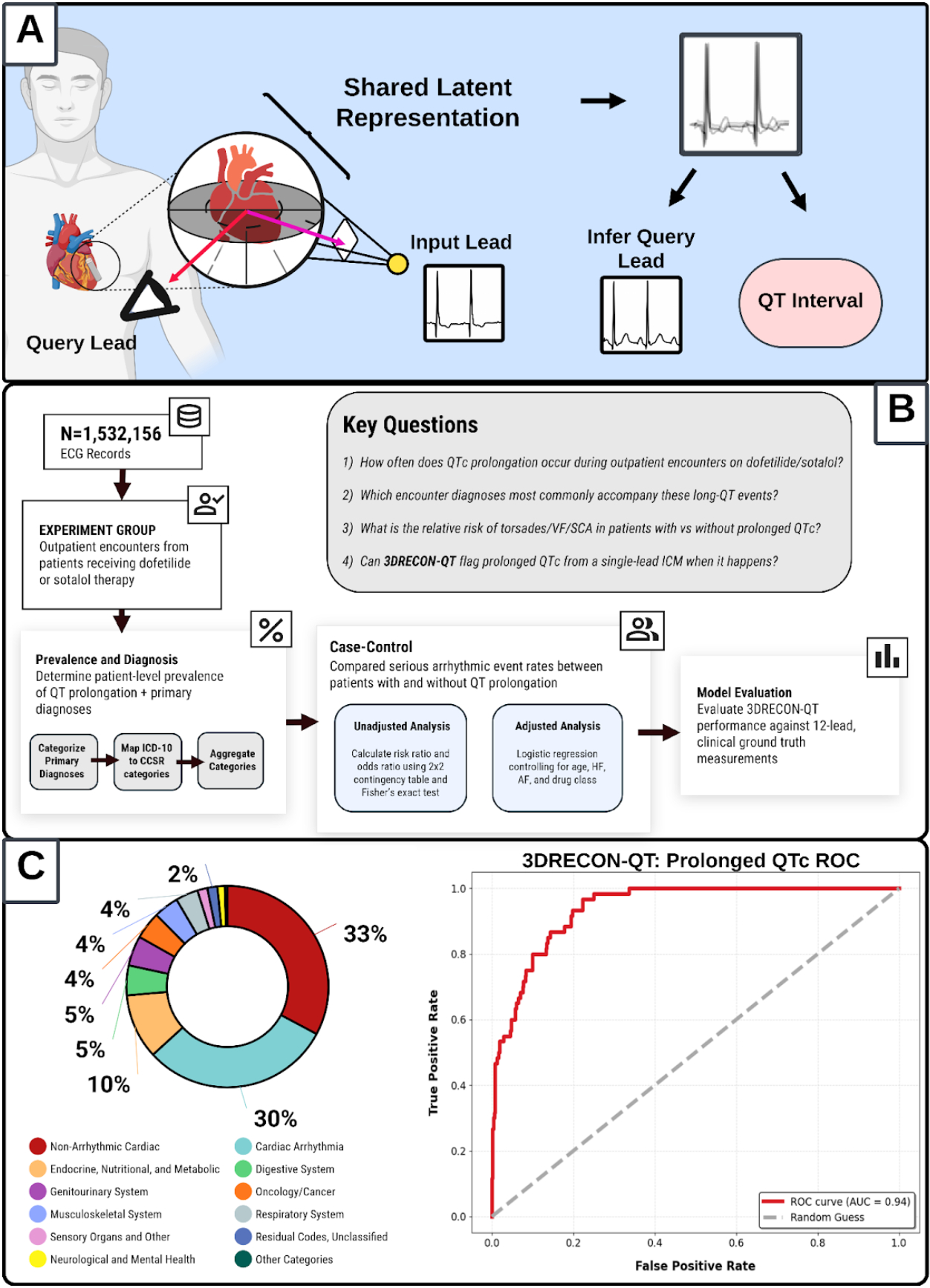
Abstract Body (Do not enter title and authors here): Background: The QT interval is a critical marker for life-threatening arrhythmic risk. Class III antiarrhythmics require inpatient QTc monitoring during initiation, but patients are discharged without continuous surveillance. While implantable cardiac monitors (ICMs) offer continuous recording, they cannot measure QTc due to the lack of standard spatial vectors. We hypothesized that outpatients experience undetected QTc prolongation with serious arrhythmic consequences, detectable by 3DRECON-QT—a spatially encoded deep-learning model developed to extract QT from single-lead ICM signals (fig. A).
Methods: We retrospectively analyzed 72,919 outpatient ECGs from 1,676 patients who were on dofetilide or sotalol across 2,083 unique outpatient encounters (Stanford, 2008–2024). We: (1) characterized the burden of outpatient QTc prolongation, (2) identified the primary diagnoses prompting outpatient visits, (3) quantified the associated arrhythmic risk, and (4) validated 3DRECON-QT’s ability to detect prolonged QTc from derived ICM signals. QTc prolongation was defined as >500 ms (narrow QRS) or >550 ms (wide QRS). Serious events included torsades, VF, and sudden cardiac death (fig. B). Analyses included prevalence, encounter diagnosis, event rates (Fisher’s exact test, multivariable regression), and NEF-QT performance assessment (Pearson correlation, AUROC, sensitivity, specificity).
Results: Despite initial inpatient drug initiation, 277/1,676 patients (16.5%) developed outpatient QT prolongation during a subsequent visit (fig. C). The 2,083 outpatient encounters presented for diverse reasons beyond arrhythmia management. At the encounter level, prolonged QTc was associated with significantly higher risk, with serious arrhythmic events occurring in 4.15% for patients presenting with prolonged-QTc vs 0.90% for normal QTc encounters (OR 4.75, p<0.05; AOR 4.24, 95% CI 1.81–9.90, p<0.05). 3DRECON-QT identified these episodes with AUROC = 0.94, sensitivity = 80%, specificity = 90%, negative predictive value = 97%, and correlation = 0.82.
Conclusions: One in six patients had documented QTc prolongation after discharge and had a fourfold increase of critical ventricular arrhythmia risk. 3DRECON-QT accurately identified these events from single-lead derived ICM signals, supporting its potential to close the outpatient surveillance gap in QT interval/risk monitoring.
Methods: We retrospectively analyzed 72,919 outpatient ECGs from 1,676 patients who were on dofetilide or sotalol across 2,083 unique outpatient encounters (Stanford, 2008–2024). We: (1) characterized the burden of outpatient QTc prolongation, (2) identified the primary diagnoses prompting outpatient visits, (3) quantified the associated arrhythmic risk, and (4) validated 3DRECON-QT’s ability to detect prolonged QTc from derived ICM signals. QTc prolongation was defined as >500 ms (narrow QRS) or >550 ms (wide QRS). Serious events included torsades, VF, and sudden cardiac death (fig. B). Analyses included prevalence, encounter diagnosis, event rates (Fisher’s exact test, multivariable regression), and NEF-QT performance assessment (Pearson correlation, AUROC, sensitivity, specificity).
Results: Despite initial inpatient drug initiation, 277/1,676 patients (16.5%) developed outpatient QT prolongation during a subsequent visit (fig. C). The 2,083 outpatient encounters presented for diverse reasons beyond arrhythmia management. At the encounter level, prolonged QTc was associated with significantly higher risk, with serious arrhythmic events occurring in 4.15% for patients presenting with prolonged-QTc vs 0.90% for normal QTc encounters (OR 4.75, p<0.05; AOR 4.24, 95% CI 1.81–9.90, p<0.05). 3DRECON-QT identified these episodes with AUROC = 0.94, sensitivity = 80%, specificity = 90%, negative predictive value = 97%, and correlation = 0.82.
Conclusions: One in six patients had documented QTc prolongation after discharge and had a fourfold increase of critical ventricular arrhythmia risk. 3DRECON-QT accurately identified these events from single-lead derived ICM signals, supporting its potential to close the outpatient surveillance gap in QT interval/risk monitoring.
More abstracts on this topic:
Addressing Racial Bias in GPT-4 Cardiovascular Clinical Reasoning
Krieger Katherine, Rossi Camilla, Rahouma Mohamed, Gaudino Mario, Hameed Irbaz, Quer Giorgio, Mack Charles, Savic Marco, Mantaj Polina, Hirofuji Aina, Gregg Alexander, Soletti Giovanni
A Culturally Tailored mHealth Lifestyle Intervention Improves Diet and Physical Activity Self-Regulation Among African AmericansLalika Mathias, Jenkins Sarah, Hayes Sharonne, Jones Clarence, Burke Lora, Cooper Lisa, Patten Christi, Brewer Laprincess