Final ID: MP803
Cardiorenal Benefits of SGLT2 Inhibitors in HFrEF Patients with ESRD Requiring Dialysis
Abstract Body (Do not enter title and authors here): Background
Sodium-glucose co-transporter 2 inhibitors (SGLT2i) have demonstrated robust cardiorenal benefits in patients with heart failure with reduced ejection fraction (HFrEF). However, their efficacy and safety in patients with end-stage renal disease (ESRD) requiring dialysis remain underexplored, as these individuals were largely excluded from pivotal clinical trials.
Methods
We conducted a retrospective cohort study using deidentified, aggregate data from the TriNetX research network. Adults aged 51-79 years with comorbid HfrEF and ESRD on dialysis were included. Patients were stratified based on SGLT2i use, with all available agents considered. Propensity score matching was applied to balance baseline characteristics. Outcomes were assessed over an 18-month (540-day) follow-up period and included acute decompensated heart failure (ADHF) events, all-cause mortality, hospitalizations, and emergency department (ED) visits. Odds ratios and Cox proportional hazards models were used to compare outcomes between groups.
Results
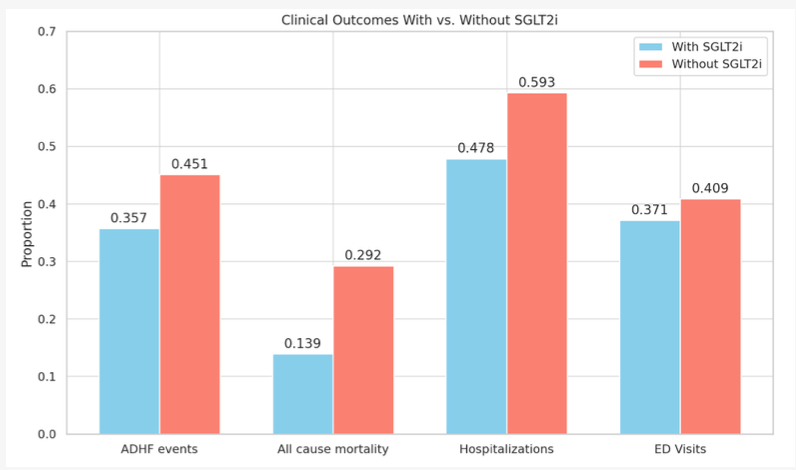
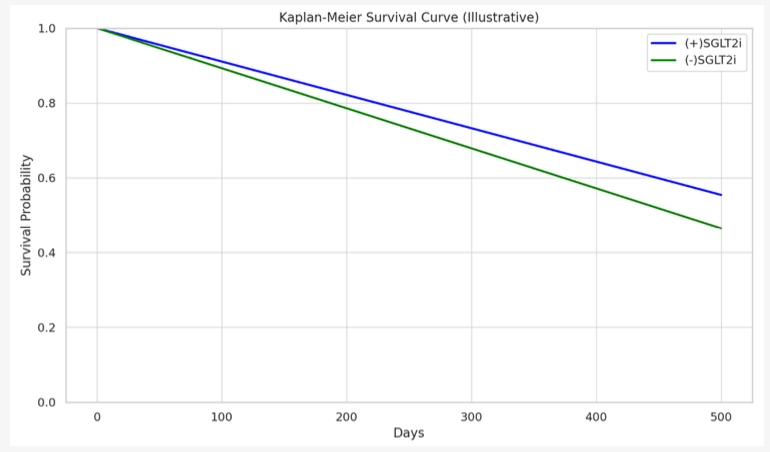
The final cohort included 8,168 patients (n = 4,084 per group; mean age 61 years; 32.5% female; 49.4% White). SGLT2i use was associated with significantly lower rates of all-cause mortality (13.9% vs. 29.2%; risk difference -15.3%, p < 0.001), ADHF events (35.7% vs. 45.1%; risk difference -9.4%, p < 0.001), hospitalizations (47.8% vs. 59.3%; risk difference -11.4%, p < 0.001), and ED visits (37.1% vs. 40.9%; risk difference -3.7%, p < 0.001). Kaplan-Meier analysis demonstrated a statistically significant improvement in survival among SGLT2i users compared to non-users (log-rank p < 0.001).
Conclusion
In patients with HFrEF and ESRD on dialysis, SGLT2i therapy was associated with significant reductions in mortality, heart failure exacerbations, and healthcare utilization. These findings support the potential role of SGLT2i in this high-risk population and underscore the need for prospective trials to confirm long-term safety and efficacy.
Sodium-glucose co-transporter 2 inhibitors (SGLT2i) have demonstrated robust cardiorenal benefits in patients with heart failure with reduced ejection fraction (HFrEF). However, their efficacy and safety in patients with end-stage renal disease (ESRD) requiring dialysis remain underexplored, as these individuals were largely excluded from pivotal clinical trials.
Methods
We conducted a retrospective cohort study using deidentified, aggregate data from the TriNetX research network. Adults aged 51-79 years with comorbid HfrEF and ESRD on dialysis were included. Patients were stratified based on SGLT2i use, with all available agents considered. Propensity score matching was applied to balance baseline characteristics. Outcomes were assessed over an 18-month (540-day) follow-up period and included acute decompensated heart failure (ADHF) events, all-cause mortality, hospitalizations, and emergency department (ED) visits. Odds ratios and Cox proportional hazards models were used to compare outcomes between groups.
Results
The final cohort included 8,168 patients (n = 4,084 per group; mean age 61 years; 32.5% female; 49.4% White). SGLT2i use was associated with significantly lower rates of all-cause mortality (13.9% vs. 29.2%; risk difference -15.3%, p < 0.001), ADHF events (35.7% vs. 45.1%; risk difference -9.4%, p < 0.001), hospitalizations (47.8% vs. 59.3%; risk difference -11.4%, p < 0.001), and ED visits (37.1% vs. 40.9%; risk difference -3.7%, p < 0.001). Kaplan-Meier analysis demonstrated a statistically significant improvement in survival among SGLT2i users compared to non-users (log-rank p < 0.001).
Conclusion
In patients with HFrEF and ESRD on dialysis, SGLT2i therapy was associated with significant reductions in mortality, heart failure exacerbations, and healthcare utilization. These findings support the potential role of SGLT2i in this high-risk population and underscore the need for prospective trials to confirm long-term safety and efficacy.
More abstracts on this topic:
Angiopoeitin-2 and Mortality in an End-Stage Renal Disease, Heart Failure Population
Robbin Vanessa, Bansal Vinod, Siddiqui Fakiha, Fareed Jawed, Syed Mushabbar
6-Nitrodopamine potentiates the positive chronotopic and inotropic effect induced by noradrenaline in the rat isolated heartLima Antonio, Sobanski Joao Fernando, Antunes Edson, De Nucci Gilberto