Final ID: MP1036
MicroRNA Biomarkers of Cardiac Allograft Vasculopathy after Heart Transplantation
Abstract Body (Do not enter title and authors here): Introduction
Cardiac allograft vasculopathy (CAV), a form of immunologically-mediated coronary disease, is a leading cause of long-term graft failure after heart transplantation (HT). MicroRNAs (miRs) biomarkers are differentially regulated during acute rejection and may predict CAV before clinical diagnosis to allow for earlier implementation of preventive strategies.
Methods
Between 2015-2023 HT patients from a single-center were enrolled and followed for the development of CAV by coronary angiography. Clinical data was extracted from United Network for Organ Sharing (UNOS) and the electronic medical record. Blood samples were collected longitudinally after transplant, and patients had small RNA extraction and high-throughput sequencing performed on plasma samples collected within 3 months of HT. The exceRpt pipeline was used to map miRs and lowly expressed miRs were excluded (< 50 average reads). Coronary angiograms were reviewed through 5-years post-transplant. A Cox regression model was used to evaluate the association between miR expression and incident CAV.
Results
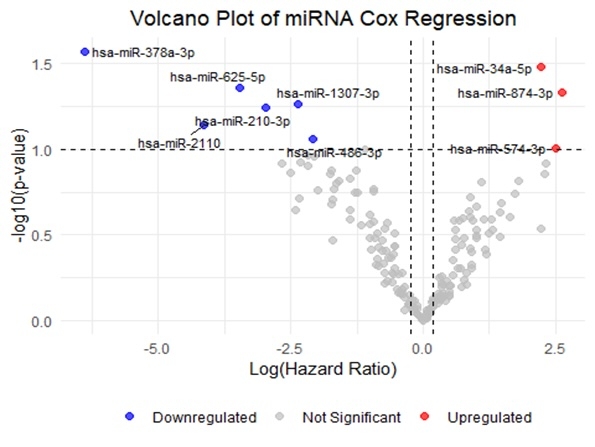
A total of 51 HT recipients were included, the median age at transplant was 54.4 years (IQR 13. 49), 72.6% were males with a racial composition of 45.1% White, 43.1% Black, and 11.8% other. Comorbidities included diabetes mellitus (33.3%) and coronary artery disease (39.2%). CAV was diagnosed in 7 of 51 patients (13.7%). Among these, 4 patients had CAV grade 1, 2 had grade 2, and 1 developed grade 3 based on coronary angiography. In multivariable analysis, patient age, donor age, higher patient BMI and male patient and donor sex were associated with development of CAV. 187 miRs were included in the analysis. 13 miRs were differentially expressed (p-value < 0.10) between patients with and without CAV(Figure). The risk of CAV varied and certain miRs were down-regulated (miR-378a-3p (HR = 0.04, 95% CI: 0.00-0.47, p = 0.011), miR-501-3p (HR = 0.06, 95% CI: 0.01-0.58, p = 0.015), and miR-625-5p (HR = 0.09, 95% CI: 0.01-0.99, p = 0.049)) and others were upregulated (miR-128-3p (HR = 9.89, 95% CI: 1.44-67.95, p = 0.02)).
Conclusion
Early post-HT, we identified a panel of circulating miRs that correlated with an increased risk of developing CAV during follow-up. With appropriate validation, these circulating miRs may identify high-risk HT patients who would benefit from early interventional strategies (e.g. conversion to an mTOR inhibitor or aggressive LDL reduction) to reduce the risk of future CAV.
Cardiac allograft vasculopathy (CAV), a form of immunologically-mediated coronary disease, is a leading cause of long-term graft failure after heart transplantation (HT). MicroRNAs (miRs) biomarkers are differentially regulated during acute rejection and may predict CAV before clinical diagnosis to allow for earlier implementation of preventive strategies.
Methods
Between 2015-2023 HT patients from a single-center were enrolled and followed for the development of CAV by coronary angiography. Clinical data was extracted from United Network for Organ Sharing (UNOS) and the electronic medical record. Blood samples were collected longitudinally after transplant, and patients had small RNA extraction and high-throughput sequencing performed on plasma samples collected within 3 months of HT. The exceRpt pipeline was used to map miRs and lowly expressed miRs were excluded (< 50 average reads). Coronary angiograms were reviewed through 5-years post-transplant. A Cox regression model was used to evaluate the association between miR expression and incident CAV.
Results
A total of 51 HT recipients were included, the median age at transplant was 54.4 years (IQR 13. 49), 72.6% were males with a racial composition of 45.1% White, 43.1% Black, and 11.8% other. Comorbidities included diabetes mellitus (33.3%) and coronary artery disease (39.2%). CAV was diagnosed in 7 of 51 patients (13.7%). Among these, 4 patients had CAV grade 1, 2 had grade 2, and 1 developed grade 3 based on coronary angiography. In multivariable analysis, patient age, donor age, higher patient BMI and male patient and donor sex were associated with development of CAV. 187 miRs were included in the analysis. 13 miRs were differentially expressed (p-value < 0.10) between patients with and without CAV(Figure). The risk of CAV varied and certain miRs were down-regulated (miR-378a-3p (HR = 0.04, 95% CI: 0.00-0.47, p = 0.011), miR-501-3p (HR = 0.06, 95% CI: 0.01-0.58, p = 0.015), and miR-625-5p (HR = 0.09, 95% CI: 0.01-0.99, p = 0.049)) and others were upregulated (miR-128-3p (HR = 9.89, 95% CI: 1.44-67.95, p = 0.02)).
Conclusion
Early post-HT, we identified a panel of circulating miRs that correlated with an increased risk of developing CAV during follow-up. With appropriate validation, these circulating miRs may identify high-risk HT patients who would benefit from early interventional strategies (e.g. conversion to an mTOR inhibitor or aggressive LDL reduction) to reduce the risk of future CAV.
More abstracts on this topic:
Allograft Outcomes of Adding Proprotein Convertase Subtilisin Kexin 9 Inhibitors or Ezetimibe to Statin Therapy in Heart Transplants Recipients at High Risk of Cardiac Allograft Progression: A Multicenter Target Trial Emulation
Hsieh Rebecca, Kumar Agara, Lee Pei-lun, Chi Kuan Yu, Tran Viet Nghi, Wang Yu-chiang
A novel small molecule synthesized based on a snail hibernation model induces hibernation in mouse fibroblasts and perfused heartsPiao Jiyuan, Zhang Yongneng, Zhao Yuan Yuan, Hannington Patrick, Tabatabaei Dakhili Seyed Amirhossein, Ussher John, Sutendra Gopinath, Michelakis Evangelos