Final ID: Su2106
Efficacy of Icosapent Ethyl for Cardiovascular Risk Reduction by Aspirin Use in REDUCE-IT
Icosapent ethyl, a purified eicosapentaenoic acid, has demonstrated CV risk reduction. Emerging evidence suggests that the antiplatelet effects of icosapent ethyl may contribute to this CV benefit.
Hypothesis:
Clinical outcomes with icosapent ethyl among patients with or without aspirin use have not been reported. This is an important evidence gap given the potential overlapping antiplatelet effects.
Methods:
REDUCE-IT was a double-blind clinical trial randomizing patients to icosapent ethyl (2g twice per day) or placebo. Statin-treated patients with elevated triglycerides (135-499 mg/dL), controlled LDL-C (41-100 mg/dL), and increased CV risk were included. In this analysis, patients with or without aspirin use were included and evaluated by randomization group. Patients were assessed overall as well as among the primary and secondary (prior CV disease) prevention cohort. The primary composite endpoint included events of nonfatal MI, nonfatal stroke, coronary revascularization, hospitalization for unstable angina, or CV death. The key secondary endpoint included events of nonfatal MI, nonfatal stroke, or CV death.
Results:
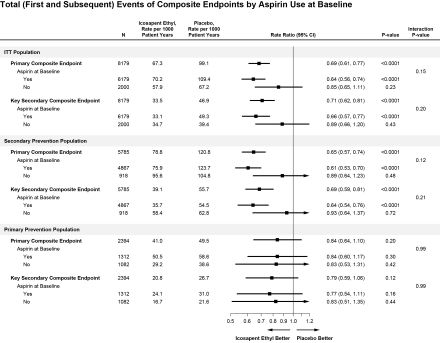
Among 8179 patients, 6179 (75.5%) were with aspirin use. Icosapent ethyl significantly reduced the primary endpoint events compared with placebo (67.3 vs 99.1 events/1000 patient-years [p-y]; Rate Ratio (RR): 0.69 [95% CI: 0.61, 0.77]; P<0.0001), with consistent effects among those with aspirin use (70.2 vs 109.4 events/1000 p-y; RR: 0.64 [95% CI: 0.56, 0.74]) or without (57.9 vs 67.2 events/1000 p-y; RR: 0.85 [95% CI: 0.65, 1.11]) (Pinteraction[int]=0.15) (Figure). Similar benefits were observed with icosapent ethyl for key secondary composite endpoint events (Pint=0.20). Findings were numerically similar in the primary prevention cohort, though not statistically significant. Among 5785 patients in the secondary prevention cohort, icosapent ethyl similarly reduced primary endpoint events (78.8 vs 120.8 events/1000 p-y; RR: 0.65 [95% CI: 0.57, 0.74]; P<0.0001), with consistency among those with aspirin use (75.9 vs 123.7 events/1000 p-y; RR: 0.61 [95% CI: 0.53, 0.70]) or without (95.6 vs 104.8 events/1000 p-y; RR: 0.89 [95% CI: 0.64, 1.23]) (Pint=0.12).
Conclusion:
Among patients with elevated triglycerides, controlled LDL, and high CV risk, icosapent ethyl reduced CV outcomes irrespective of aspirin use. These findings suggest icosapent ethyl has CV benefit incremental to concomitant background therapy with statins plus aspirin.
- Aggarwal, Rahul ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Steg, Philippe ( Hopital Bichat , Paris , France )
- Bhatt, Deepak ( Mount Sinai Fuster Heart Hospital , Scarsdale , New York , United States )
- Ballantyne, Christie ( BAYLOR COLLEGE MEDICINE , Houston , Texas , United States )
- Miller, Michael ( Philadelphia VA-Univ Penn , Philadelphia , Pennsylvania , United States )
- Brinton, Eliot ( Utah Lipid Center , Salt Lake City , Utah , United States )
- Jacobson, Terry ( EMORY UNIVERSITY , Atlanta , Georgia , United States )
- Ketchum, Steven ( Amarin Pharma Inc. , Bridgewater , New Jersey , United States )
- Doyle, Ralph ( Amarin Pharma Inc. , Bedminster , New Jersey , United States )
- Tardif, Jean-claude ( MONTREAL HEART INSTITUTE , Montreal , Quebec , Canada )
Meeting Info:
Session Info:
After the Infarct: Trends, Treatments, and Missed Opportunities
Sunday, 11/09/2025 , 03:15PM - 04:15PM
Abstract Poster Board Session
More abstracts on this topic:
Liu Jing, Geng Jialu, Liu Jiakun, Hu Lei, Du Huimin, Hung Ivan Tjong A, Fang Jia
DR10624, a First-In-Class, FGF21 Receptor/Glucagon Receptor/GLP-1 Receptor Triple Agonist, Rapidly and Significantly Reduced Triglycerides, Atherogenic Lipids, and Liver Fat in Patients With Severe Hypertriglyceridemia: Primary Results From a Randomized Phase 2 Trial.Li Jianping, Zhou Zijian, Lv Lingchun, Gan Yulong, Wang Ying, Zhu Chaonan, Xu June, Huang Yanshan, Fang Yongliang, Zhang Long, Fan Yanting, Zhang Shu, Wang Liyun, Qu Yanling, Yin Guotian, Jiang Hongwei
More abstracts from these authors:
Tardif Jean-claude, Dweck Marc
Substantial cardiovascular risk reduction with icosapent ethyl in patients with prior cardiovascular events regardless of coronary artery disease history: REDUCE-IT CADAggarwal Rahul, Tardif Jean-claude, Steg Philippe, Bhatt Deepak, Ballantyne Christie, Miller Michael, Brinton Eliot, Jacobson Terry, Ketchum Steven, Lira Pineda Armando, Doyle Ralph