Final ID: Mo2050
Real-World Effectiveness of GLP-1 Receptor Agonists on Clinical Outcomes in Patients with Heart Failure with Preserved Ejection Fraction (HFpEF).
Abstract Body (Do not enter title and authors here): Introduction/Background: Multiple clinical trials have demonstrated that glucagon-like peptide 1 receptor agonists (GLP-1 Ras) improve symptoms, physical function, and potentially reduce hospitalizations in selected populations with HFpEF. However, the effectiveness of GLP-1 RAs in real-world populations with HFpEF is not well-characterized.
Research Questions/Hypothesis: To evaluate the association between GLP-1 RA use and clinical outcomes in patients with HFpEF using real-world data.
Methods/Approach: We used a target trial emulation framework and Truveta data to evaluate real-world GLP-1 effectiveness in new users with a HFpEF encounter from 2009 to 2024. Patients with HFpEF were included in two cohorts based on first-time initiation of (1) antiobesity medications (AOM) in individuals with or without obesity, and (2) antidiabetes medications (ADM) in individuals with or without diabetes. Overlap was possible, but each cohort was analyzed independently to address different clinical contexts. Our primary outcome was time to first occurrence of a composite endpoint (HF hospitalization, urgent HF encounter, or CV death within 1 year). Cox proportional hazards models were used to estimate the relative event hazards for GLP-1 RA users vs. several comparator groups for AOM [bupropion-naltrexone (BN), phentermine-topiramate (PT)] and ADM [DPP4i and SGLT2i].
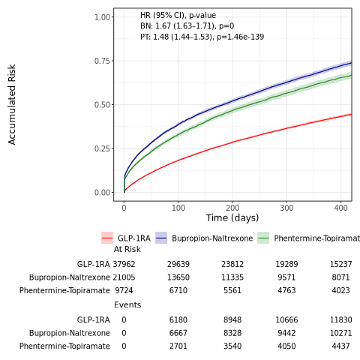
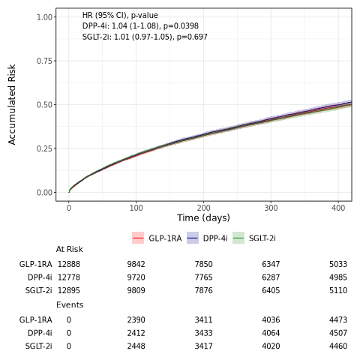
Results/Data: Of 107,252 patients with HFpEF, 68,691 patients (64.0%) were included in the AOM subset [(mean BMI 36.7; 37,962 on GLP1RA (55.3%)], and 38,561 patients (36.0%) met criteria for ADM cohort [12,888 on GLP1RA (33.4%)]. In the AOM population, the unadjusted hazard of the composite endpoint was lower in the GLP-1RA population in comparison to the BN [HR 1.67 (95% CI: 1.63-1.71), p = 0] and PT [HR 1.48 (95% CI: 1.44-1.53), p <0.0001] populations over the one-year follow-up period (Figure 1). In the ADM population, the unadjusted hazard rates were similar across GLP-1RA, SGLT-2i [HR =1.01 (95% CI: 0.97-1.05), p = 0.697], and DPP-4i [HR = 1.04 (95% CI: 1.0-1.08), p = 0.0398] users over the one year (Figure 2). Analyses of fully adjusted models are ongoing and will be presented at the meeting.
Conclusions: Among patients with HFpEF, the use of GLP-1 RA in the context of obesity treatment was associated with a reduced unadjusted incidence of cardiovascular events, suggesting that efficacy observed in clinical trials may generalize to effectiveness in routine clinical practice.
Research Questions/Hypothesis: To evaluate the association between GLP-1 RA use and clinical outcomes in patients with HFpEF using real-world data.
Methods/Approach: We used a target trial emulation framework and Truveta data to evaluate real-world GLP-1 effectiveness in new users with a HFpEF encounter from 2009 to 2024. Patients with HFpEF were included in two cohorts based on first-time initiation of (1) antiobesity medications (AOM) in individuals with or without obesity, and (2) antidiabetes medications (ADM) in individuals with or without diabetes. Overlap was possible, but each cohort was analyzed independently to address different clinical contexts. Our primary outcome was time to first occurrence of a composite endpoint (HF hospitalization, urgent HF encounter, or CV death within 1 year). Cox proportional hazards models were used to estimate the relative event hazards for GLP-1 RA users vs. several comparator groups for AOM [bupropion-naltrexone (BN), phentermine-topiramate (PT)] and ADM [DPP4i and SGLT2i].
Results/Data: Of 107,252 patients with HFpEF, 68,691 patients (64.0%) were included in the AOM subset [(mean BMI 36.7; 37,962 on GLP1RA (55.3%)], and 38,561 patients (36.0%) met criteria for ADM cohort [12,888 on GLP1RA (33.4%)]. In the AOM population, the unadjusted hazard of the composite endpoint was lower in the GLP-1RA population in comparison to the BN [HR 1.67 (95% CI: 1.63-1.71), p = 0] and PT [HR 1.48 (95% CI: 1.44-1.53), p <0.0001] populations over the one-year follow-up period (Figure 1). In the ADM population, the unadjusted hazard rates were similar across GLP-1RA, SGLT-2i [HR =1.01 (95% CI: 0.97-1.05), p = 0.697], and DPP-4i [HR = 1.04 (95% CI: 1.0-1.08), p = 0.0398] users over the one year (Figure 2). Analyses of fully adjusted models are ongoing and will be presented at the meeting.
Conclusions: Among patients with HFpEF, the use of GLP-1 RA in the context of obesity treatment was associated with a reduced unadjusted incidence of cardiovascular events, suggesting that efficacy observed in clinical trials may generalize to effectiveness in routine clinical practice.
More abstracts on this topic:
A Highly Selective and Orally Available HDAC6 Inhibitor, EKZ-102, Ameliorates Cardiac Dysfunction and Exercise Intolerance in Cardiometabolic HFpEF
Elbatreek Mahmoud, Goodchild Traci, Lefer David, Evans Lauren, Richardson Thomas, James Rebecca, Schroeder Frederick, Wang Jianhong, Luterman Jim, Gilbert Tonya, Fisher Richard
A Case of Steroid-Refractory Immune-checkpoint-inhibitor Induced Myocarditis Responsive to Mycophenolate and Anti-thymocyte globulinDabdoub Jorge, Wilson Michael, Gottbrecht Matthew, Salazar Ryan, Shih Jeffrey