Final ID: MP1811
Association Between Genetics Variants And Clinical Outcomes In Patients With Left Ventricular Assist Device
Abstract Body (Do not enter title and authors here): Introduction
Right ventricular failure (RVF) is a common complication following left ventricular assist device (LVAD) implantation, resulting in significant morbidity and mortality. However, accurate prediction of RVF after LVAD remains limited, highlighting a critical need to improve patient selection and identify those who may benefit from enhanced right ventricular (RV) support.
Hypothesis
We hypothesized that patients with genetic cardiomyopathies exhibit global myocardial dysfunction, including RV impairment and are at higher risk of RVF following LVAD implantation.
Methods
We conducted a single-center retrospective study of consecutive adult patients who underwent LVAD implantation from January 1, 2018, to June 30, 2024. Clinical and genetic data were collected from the electronic medical record. Post-LVAD outcomes were classified using the 2021 INTERMACS Adverse Event Definitions, with early RVF defined as occurring within 30 days of implantation.
Results
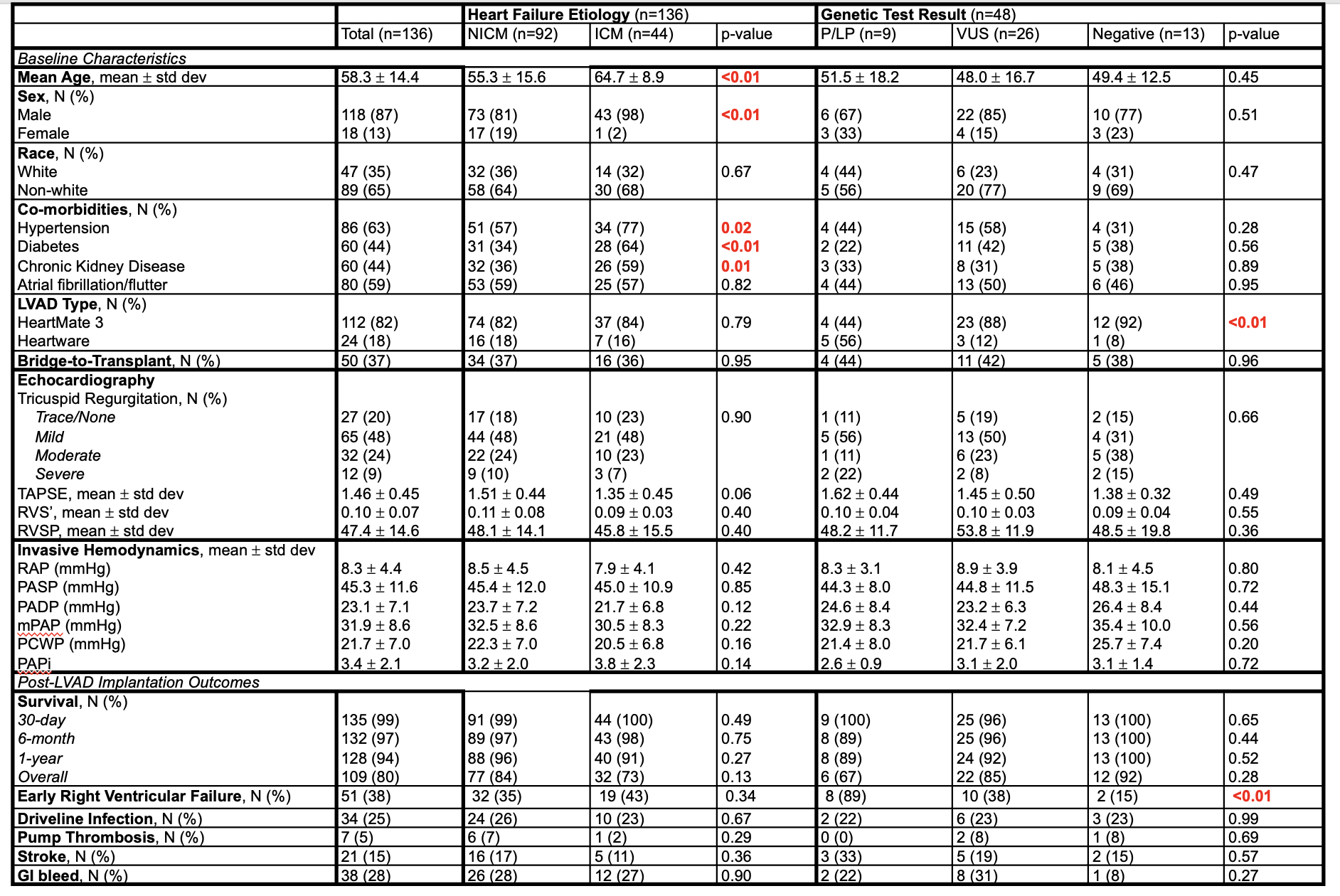
Among 136 LVAD recipients (87% male, 65% non-White, mean age 58 ± 14.4 years), most had non-ischemic cardiomyopathy (NICM, 68%) and received HeartMate 3 devices (82%) as destination therapy (63%). No significant differences in post-LVAD outcomes were observed between NICM and ischemic cardiomyopathy (ICM) groups. Genetic testing was performed in 48 patients (35%), revealing pathogenic/likely pathogenic (P/LP) variants in 9 (19%), variants of uncertain significance (VUS) in 26 (54%), and negative results in 13 (27%). Baseline RV function by echocardiography and invasive hemodynamics did not differ by genetic test results. Furthermore, genetic test results were not associated with significant differences in post-LVAD outcomes, including survival, driveline infection, pump thrombosis, stroke, and gastrointestinal bleeding. However, patients with P/LP variants had a significantly higher incidence of early RVF compared to those with VUS or negative results (89% vs. 38% vs. 15%, p<0.01). Among only HeartMate 3 recipients, early RVF remained significantly more common in those with P/LP variants (75% vs. 30% vs. 8.3%, p=0.035).
Conclusion
Presence of clinically actionable genetic (P/LP) variants were significantly associated with a higher incidence of early RVF post-LVAD. There were no differences in outcomes when stratified by cardiomyopathy etiology. These findings suggest that genetic testing may help identify patients who are at higher risk of early RVF post-LVAD and inform perioperative management strategies.
Right ventricular failure (RVF) is a common complication following left ventricular assist device (LVAD) implantation, resulting in significant morbidity and mortality. However, accurate prediction of RVF after LVAD remains limited, highlighting a critical need to improve patient selection and identify those who may benefit from enhanced right ventricular (RV) support.
Hypothesis
We hypothesized that patients with genetic cardiomyopathies exhibit global myocardial dysfunction, including RV impairment and are at higher risk of RVF following LVAD implantation.
Methods
We conducted a single-center retrospective study of consecutive adult patients who underwent LVAD implantation from January 1, 2018, to June 30, 2024. Clinical and genetic data were collected from the electronic medical record. Post-LVAD outcomes were classified using the 2021 INTERMACS Adverse Event Definitions, with early RVF defined as occurring within 30 days of implantation.
Results
Among 136 LVAD recipients (87% male, 65% non-White, mean age 58 ± 14.4 years), most had non-ischemic cardiomyopathy (NICM, 68%) and received HeartMate 3 devices (82%) as destination therapy (63%). No significant differences in post-LVAD outcomes were observed between NICM and ischemic cardiomyopathy (ICM) groups. Genetic testing was performed in 48 patients (35%), revealing pathogenic/likely pathogenic (P/LP) variants in 9 (19%), variants of uncertain significance (VUS) in 26 (54%), and negative results in 13 (27%). Baseline RV function by echocardiography and invasive hemodynamics did not differ by genetic test results. Furthermore, genetic test results were not associated with significant differences in post-LVAD outcomes, including survival, driveline infection, pump thrombosis, stroke, and gastrointestinal bleeding. However, patients with P/LP variants had a significantly higher incidence of early RVF compared to those with VUS or negative results (89% vs. 38% vs. 15%, p<0.01). Among only HeartMate 3 recipients, early RVF remained significantly more common in those with P/LP variants (75% vs. 30% vs. 8.3%, p=0.035).
Conclusion
Presence of clinically actionable genetic (P/LP) variants were significantly associated with a higher incidence of early RVF post-LVAD. There were no differences in outcomes when stratified by cardiomyopathy etiology. These findings suggest that genetic testing may help identify patients who are at higher risk of early RVF post-LVAD and inform perioperative management strategies.
More abstracts on this topic:
Advanced Machine Learning Models for Classifying Transthyretin Amyloidosis in Clinical Settings
Verma Anurag, Hsu Po-ya, Kripke Colleen, Howard William, Sirugo Giorgio, Myes Kelly
Acute Hemodynamic Effects of Sotatercept in Pulmonary Arterial HypertensionKremer Nils, Naeije Robert, Ghofrani Ardeschir, Tello Khodr, Thal Bruno, Janetzko Patrick, Yogeswaran Athiththan, Rako Zvonimir, Pullamsetti Soni, Bonnet Sebastien, Seeger Werner, Grimminger Friedrich