Final ID: MP2628
Exogenous Ketone Supplementation Restores Length-Dependent Activation In Human Hypertrophic Cardiomyopathy Myocardium
Abstract Body (Do not enter title and authors here): Introduction: Metabolic dysfunction in hypertrophic cardiomyopathy (HCM) is thought to both contribute to and result from disease progression. Small trials of therapeutic ketosis in heart failure demonstrated acute improvements in cardiac function and reduced systemic vascular resistance. Given the known vasodilatory effects of ketones, it is unclear whether improvements in cardiac function were due to reduced afterload, direct myocardial effect, or both. We used human living myocardial slices (LMS) to examine the direct impact of exogenous ketones on myocardial work under a variety of loading conditions to study load-dependent effects of both disease and fuel.
Hypothesis: Ketone supplementation acutely and directly alters length-dependent activation in HCM independent from afterload.
Methods: Vibratome-generated 300µm-thick slices of left ventricular or septal tissue from non-failing (NF) donor hearts (N=15) and HCM septal myectomy tissue (N=13) were adhered to 8x8mm anchors and mounted onto the IonOptix Cardiac Slice System in either glucose-only or glucose+ketone (6mM) solution. We generated force-length work loops at varying preloads (4-16% strain) and afterloads (25-75% active force). Data were analyzed using a mixed effects linear model with random intercepts for heart and slice.
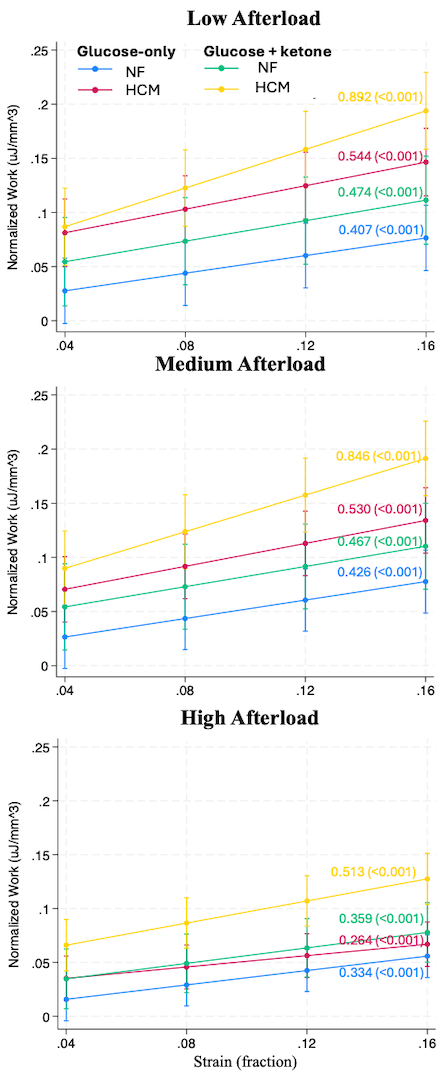
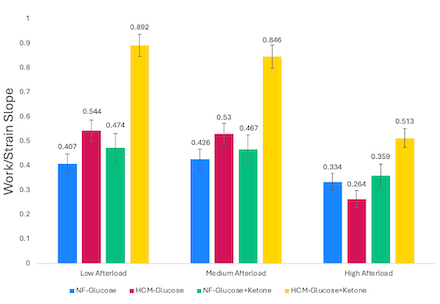
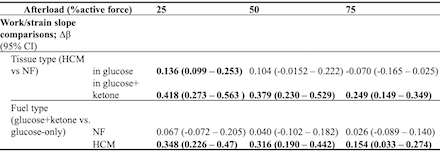
Results: At low afterload, HCM LMS in glucose had enhanced length-dependent activation compared to NF (between-group comparison of work/strain slopes (Δβ)=0.136, CI 0.099–0.253). This difference decreased at medium and high afterload (Figure 1-2, Table 1). Ketone supplementation enhanced length-dependent activation in HCM tissue across loads, with greatest impact at low afterload (Δβ=0.348, CI 0.226–0.47). HCM tissue in glucose+ketone had enhanced length-dependent activation compared to NF at all loads including high afterload (Δβ=0.249, CI 0.149–0.349) (Figure 1-2, Table 1).
Conclusion: Our LMS platform leveraged precise control over preload and afterload to recapitulate classical Frank-Starling physiology and disease-related effects. HCM tissue in glucose-only demonstrated impaired preload responsiveness at high afterload. Ketone supplementation augmented length-dependent activation in HCM tissue across afterloads, indicating direct myocardial impact of ketosis independent of afterload. However, the impact was greatest at low afterload, indicating that decreased SVR and direct myocardial effects may work cooperatively to improve cardiac function.
Hypothesis: Ketone supplementation acutely and directly alters length-dependent activation in HCM independent from afterload.
Methods: Vibratome-generated 300µm-thick slices of left ventricular or septal tissue from non-failing (NF) donor hearts (N=15) and HCM septal myectomy tissue (N=13) were adhered to 8x8mm anchors and mounted onto the IonOptix Cardiac Slice System in either glucose-only or glucose+ketone (6mM) solution. We generated force-length work loops at varying preloads (4-16% strain) and afterloads (25-75% active force). Data were analyzed using a mixed effects linear model with random intercepts for heart and slice.
Results: At low afterload, HCM LMS in glucose had enhanced length-dependent activation compared to NF (between-group comparison of work/strain slopes (Δβ)=0.136, CI 0.099–0.253). This difference decreased at medium and high afterload (Figure 1-2, Table 1). Ketone supplementation enhanced length-dependent activation in HCM tissue across loads, with greatest impact at low afterload (Δβ=0.348, CI 0.226–0.47). HCM tissue in glucose+ketone had enhanced length-dependent activation compared to NF at all loads including high afterload (Δβ=0.249, CI 0.149–0.349) (Figure 1-2, Table 1).
Conclusion: Our LMS platform leveraged precise control over preload and afterload to recapitulate classical Frank-Starling physiology and disease-related effects. HCM tissue in glucose-only demonstrated impaired preload responsiveness at high afterload. Ketone supplementation augmented length-dependent activation in HCM tissue across afterloads, indicating direct myocardial impact of ketosis independent of afterload. However, the impact was greatest at low afterload, indicating that decreased SVR and direct myocardial effects may work cooperatively to improve cardiac function.
More abstracts on this topic:
Adeno-associated virus-mediated gene delivery of PERM1 enhances cardiac contractility and mitochondrial biogenesis in mice.
Sreedevi Karthi, Doku Abigail Oforiwaa, James Amina, Do Sara, Zaitsev Alexey, Warren Junco
Desensitization of the Cardiac Troponin Complex by TnI Phosphorylation and Epigallocatechin-3-gallateTigro Helene, Solis Christopher