Final ID: Sa4003
Comparative Safety and Efficacy of Acoramidis Versus Tafamidis in Transthyretin Amyloid Cardiomyopathy: A Systematic Review
Abstract Body (Do not enter title and authors here): Background: Transthyretin amyloid cardiomyopathy (ATTR-CM) is an under-diagnosed and life-threatening
progressive infiltrative cardiomyopathy characterized by the abnormal deposition of misfolded
transthyretin (TTR) protein in the myocardium. The therapeutic options have traditionally been limited. Tafamidis is the current standard of care for transthyretin amyloid cardiomyopathy (ATTR-CM) , but there is a newer transthyretin stabilizer, Acoramidis, emerging as a potential candidate. In this review, the safety and efficacy profiles of Acoramidis are compared and evaluated against Tafamidis.
Methods: We searched PubMed and Google Scholar from inception to May 2025 for RCTs and observational trials assessing Tafamidis or Acoramidis in ATTR-CM. Primary outcomes included all-cause mortality, cardiovascular hospitalization, 6-minute walk distance (6MWD), N-terminal pro B-type natriuretic peptide (NT-proBNP), and treatment-emergent adverse events.The PRISMA stratergy is used.
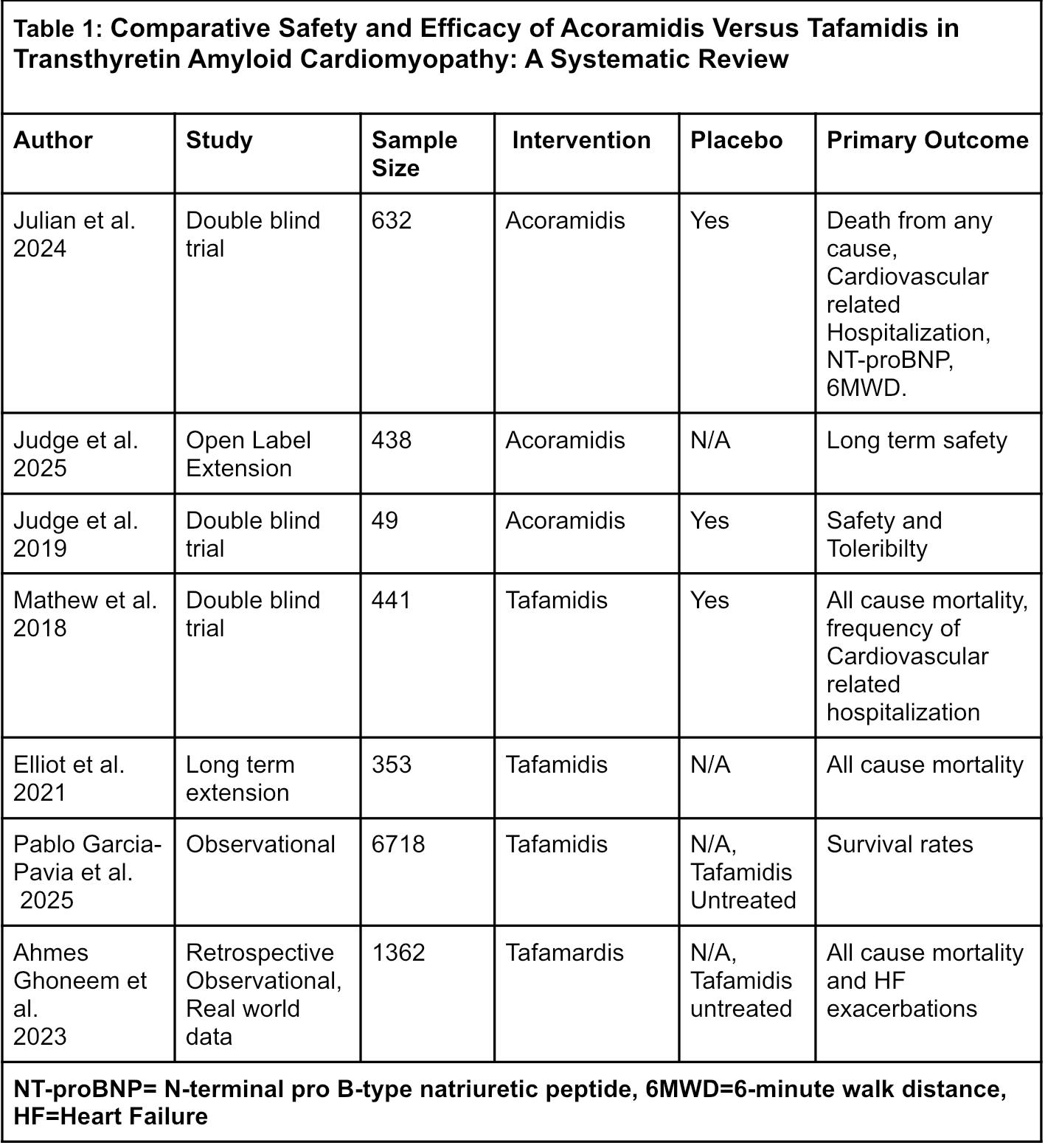
Results: Seven studies were included after screening the studies, from a comprehensive PRISMA-guided literature search. Acoramidis, in the ATTRibute-CM Phase 3 trial, demonstrated substantial clinical improvement, with a hazard ratio (HR) of 0.65 (95% CI: 0.50–0.83; p = 0.0008) for composite of all-cause mortality (ACM) and cardiovascular hospitalization (CVH), and 39.6 meters improvement in 6MWD (p < 0.001). NT-proBNP reduced substantially with a factor change of 0.529 (95% CI: 0.46–0.60), and TEAEs were similar between groups, with no significant increase in serious adverse events compared to placebo; the most common events included peripheral edema and gastrointestinal symptoms. Tafamidis, based on the ATTR-ACT trial results, was associated with an ACM HR of 0.70 (95% CI: 0.51–0.96; p = 0.0259) and reduced CVH events (RR 0.68; p < 0.0001) and enhanced 6MWD.TEAEs with Tafamidis were comparable to placebo; fatigue and urinary tract infections were among the most frequent events.
Conclusion: Both Acoramidis and Tafamidis are quite effective in ATTR-CM. Acoramidis may generate more meaningful decreases in cardiovascular hospitalizations and biomarker improvements, with a positive trend in survival at extended terms. Comparative head-to-head trials are needed to determine comparative superiority.
Keywords:
Acoramidis, Tafamidis, ATTR-CM, Transthyretin Cardiomyopathy, All cause mortality, 6-minute walk distance, Cardiovascular Outcomes, Systematic review, Cardiology
progressive infiltrative cardiomyopathy characterized by the abnormal deposition of misfolded
transthyretin (TTR) protein in the myocardium. The therapeutic options have traditionally been limited. Tafamidis is the current standard of care for transthyretin amyloid cardiomyopathy (ATTR-CM) , but there is a newer transthyretin stabilizer, Acoramidis, emerging as a potential candidate. In this review, the safety and efficacy profiles of Acoramidis are compared and evaluated against Tafamidis.
Methods: We searched PubMed and Google Scholar from inception to May 2025 for RCTs and observational trials assessing Tafamidis or Acoramidis in ATTR-CM. Primary outcomes included all-cause mortality, cardiovascular hospitalization, 6-minute walk distance (6MWD), N-terminal pro B-type natriuretic peptide (NT-proBNP), and treatment-emergent adverse events.The PRISMA stratergy is used.
Results: Seven studies were included after screening the studies, from a comprehensive PRISMA-guided literature search. Acoramidis, in the ATTRibute-CM Phase 3 trial, demonstrated substantial clinical improvement, with a hazard ratio (HR) of 0.65 (95% CI: 0.50–0.83; p = 0.0008) for composite of all-cause mortality (ACM) and cardiovascular hospitalization (CVH), and 39.6 meters improvement in 6MWD (p < 0.001). NT-proBNP reduced substantially with a factor change of 0.529 (95% CI: 0.46–0.60), and TEAEs were similar between groups, with no significant increase in serious adverse events compared to placebo; the most common events included peripheral edema and gastrointestinal symptoms. Tafamidis, based on the ATTR-ACT trial results, was associated with an ACM HR of 0.70 (95% CI: 0.51–0.96; p = 0.0259) and reduced CVH events (RR 0.68; p < 0.0001) and enhanced 6MWD.TEAEs with Tafamidis were comparable to placebo; fatigue and urinary tract infections were among the most frequent events.
Conclusion: Both Acoramidis and Tafamidis are quite effective in ATTR-CM. Acoramidis may generate more meaningful decreases in cardiovascular hospitalizations and biomarker improvements, with a positive trend in survival at extended terms. Comparative head-to-head trials are needed to determine comparative superiority.
Keywords:
Acoramidis, Tafamidis, ATTR-CM, Transthyretin Cardiomyopathy, All cause mortality, 6-minute walk distance, Cardiovascular Outcomes, Systematic review, Cardiology
More abstracts on this topic:
A Machine Learning-Derived Socio-Environmental Risk Score More Accurately Predicts Cardiovascular Events and Better Addresses Health Inequities than Social Deprivation Index
Chen Zhuo, Nasir Khurram, Al-kindi Sadeer, Rajagopalan Sanjay, Ponnana Sai Rahul, Dazard Jean-eudes, Zhang Tong, Dong Weichuan, Okyere Robert, Sirasapalli Santosh, Deo Salil, Khraishah Haitham
A Comprehensive Study on Machine Learning Models Combining with Oversampling for One-year Persistent Coronary Artery Aneurysm in Kawasaki DiseaseLiang Kaizhi, Pang Yusheng, Su Danyan