Final ID: Su4068
LARP6 Regulates Skeletal Muscle Fibrotic Response in Congestive Heart Failure
Abstract Body (Do not enter title and authors here): Introduction: Although the extracellular matrix (ECM) is essential in maintaining the structural framework of skeletal muscle, its excessive accumulation (fibrosis), especially collagens, impairs muscle function. We have found increased skeletal muscle fibrosis in congestive heart failure (CHF) conditions, likely leading to impaired muscle function.
Hypothesis: LARP6 is an mRNA binding protein that coordinates efficient translation of Collagen I α chains Col1a1 and Col1a2. We hypothesized that LARP6 regulates collagen synthesis during skeletal muscle fibrotic response in CHF conditions.
Aims: The study aims to demonstrate that LARP6 induces skeletal muscle fibrosis in CHF conditions, and that skeletal muscle fibrosis is prevented by inhibition of LARP6.
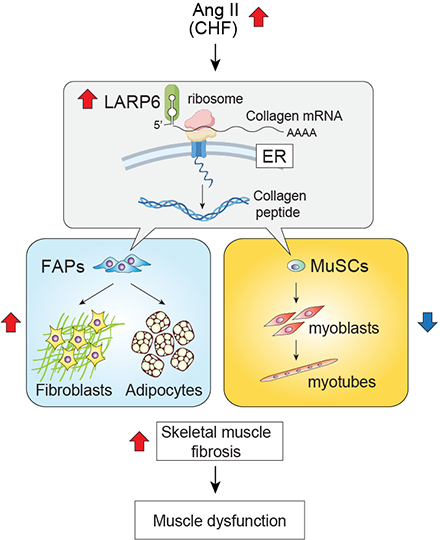
Approach: Skeletal muscle fibrosis was analyzed in vivo in two animal models relevant to CHF, angiotensin II (Ang II) infusion and left anterior descending artery (LAD) ligation. Expression of Collagen I, Larp6 and myogenic markers (myogenin and Myh3) were analyzed by qRT-PCR, western blotting, immunostaining and RNAScope (in situ mRNA detection) in hindlimb muscles in vivo and primary myoblasts and fibro/adipogenic progenitor (FAP) cells in vitro. Collagen I levels were also analyzed by picrosirius red staining (PSR). We have generated Larp6 knockout mice, and these analyses were performed in these animals.
Results: In both Ang II infusion and LAD ligation models, skeletal muscle Collagen I and LARP6 levels were increased. Among the cells in skeletal muscle, single cell RNASeq data analysis revealed that LARP6 is predominantly expressed in myoblasts and FAP populations. In cultured myoblasts and FAPs, LARP6 protein was co-localized with Col1a1 mRNA at endoplasmic reticulum (ER), and fibrotic stimulus (TGF-β treatment) increased their ER localization and Collagen I production. Importantly, deletion of Larp6 suppressed Collagen I induction in vitro and reduced Ang II-induced skeletal muscle fibrosis in vivo, associated with reduced Col1a1 mRNA localization to ER.
Conclusions: These data suggest that LARP6 regulates Collagen I protein synthesis and its filament assembly in ER likely by binding to Col1a1 mRNA. Suppression of LARP6 may be a therapeutic strategy to prevent pathophysiological fibrosis development in skeletal muscle.
Hypothesis: LARP6 is an mRNA binding protein that coordinates efficient translation of Collagen I α chains Col1a1 and Col1a2. We hypothesized that LARP6 regulates collagen synthesis during skeletal muscle fibrotic response in CHF conditions.
Aims: The study aims to demonstrate that LARP6 induces skeletal muscle fibrosis in CHF conditions, and that skeletal muscle fibrosis is prevented by inhibition of LARP6.
Approach: Skeletal muscle fibrosis was analyzed in vivo in two animal models relevant to CHF, angiotensin II (Ang II) infusion and left anterior descending artery (LAD) ligation. Expression of Collagen I, Larp6 and myogenic markers (myogenin and Myh3) were analyzed by qRT-PCR, western blotting, immunostaining and RNAScope (in situ mRNA detection) in hindlimb muscles in vivo and primary myoblasts and fibro/adipogenic progenitor (FAP) cells in vitro. Collagen I levels were also analyzed by picrosirius red staining (PSR). We have generated Larp6 knockout mice, and these analyses were performed in these animals.
Results: In both Ang II infusion and LAD ligation models, skeletal muscle Collagen I and LARP6 levels were increased. Among the cells in skeletal muscle, single cell RNASeq data analysis revealed that LARP6 is predominantly expressed in myoblasts and FAP populations. In cultured myoblasts and FAPs, LARP6 protein was co-localized with Col1a1 mRNA at endoplasmic reticulum (ER), and fibrotic stimulus (TGF-β treatment) increased their ER localization and Collagen I production. Importantly, deletion of Larp6 suppressed Collagen I induction in vitro and reduced Ang II-induced skeletal muscle fibrosis in vivo, associated with reduced Col1a1 mRNA localization to ER.
Conclusions: These data suggest that LARP6 regulates Collagen I protein synthesis and its filament assembly in ER likely by binding to Col1a1 mRNA. Suppression of LARP6 may be a therapeutic strategy to prevent pathophysiological fibrosis development in skeletal muscle.
More abstracts on this topic:
A Case of Steroid-Refractory Immune-checkpoint-inhibitor Induced Myocarditis Responsive to Mycophenolate and Anti-thymocyte globulin
Dabdoub Jorge, Wilson Michael, Gottbrecht Matthew, Salazar Ryan, Shih Jeffrey
β1-adrenergic autoantibodies (β1-AA) augment macropinocytosis in CD4+ T cells, leading to the expansion of CD4+CD28− T cell subsets in heart failure.Sun Fei, Yao Junyan, Li Bingjie, Zhang Suli, Liu Huirong