Final ID: MP1666
Impact of Cardiac Immune-Related Adverse Events on One-Year Survival in Patients Treated with Immune Checkpoint Inhibitors: A Multicenter Propensity-Matched Analysis
Abstract Body (Do not enter title and authors here): Background:
Immune checkpoint inhibitors (ICIs) are central to modern cancer immunotherapy but may lead to cardiac immune-related adverse events (irAEs), including myocarditis, pericarditis, pericardial effusion, and tamponade. These complications, while rare, may significantly impact survival, yet contemporary real-world data on outcomes are limited.
Hypothesis:
Patients who develop cardiac irAEs after ICI initiation have worse one-year survival than those who do not.
Methods:
We performed a retrospective cohort study using the TriNetX US Collaborative Network, a federated database of electronic health records from multiple healthcare organizations (HCOs). We queried data from 57 HCOs. Adult cancer patients (≥18 years) receiving any ICI between January 1st, 2012 and December 31st, 2023 were included. Two cohorts were defined: those who developed cardiac irAEs and those who did not. Cardiac irAEs were identified using ICD-10-CM codes for myocarditis, pericarditis, pericardial effusion, and tamponade. Propensity score matching (1:1) was applied based on age, sex, race/ethnicity, cancer type, cardiovascular risk factors, and baseline comorbidities. The primary outcome was one-year all-cause mortality, assessed using Kaplan-Meier analysis.
Results:
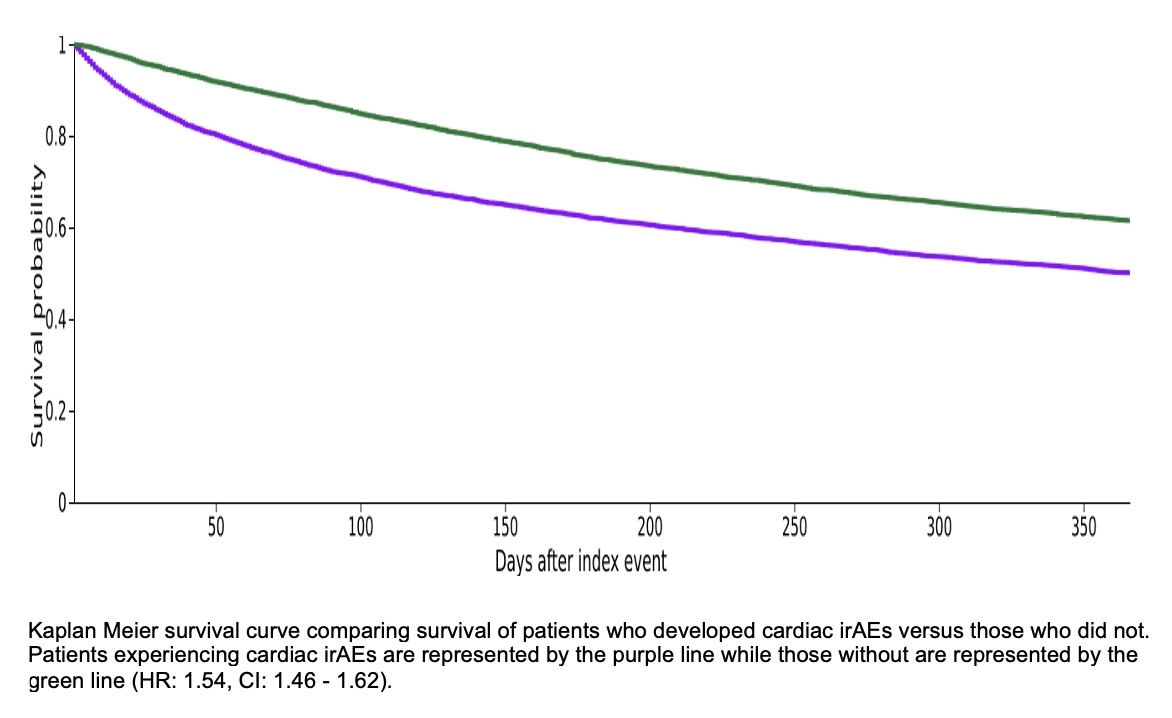
Of 109,392 eligible patients, 6,925 (6.3%) developed cardiac irAEs and 102,467 did not. After matching and excluding those who died before the follow up period, 6,610 patients remained in the irAE cohort and 6,704 in the control group. The matched population had a mean age of 68.7 ± 12.7 years; 51.6% were male, 74.2% White, 11.3% Black, 4.4% Asian, and 5.2% Hispanic/Latino. Cardiovascular and oncologic comorbidities were well balanced. One-year mortality was significantly higher among those with cardiac irAEs (3,029/6,610; 45.8%) vs. controls (2,437/6,704; 36.4%), with a risk difference of 9.5% (95% CI: 7.8–11.1; p < 0.001). One-year survival probability was 50.11% in the irAE group vs. 61.51% in controls. The hazard ratio for death was 1.54 (95% CI: 1.46–1.62; p < 0.001).
Conclusion:
Cardiac irAEs following ICI therapy are linked to significantly worse one-year survival, with a 54% higher risk of death at any given time in the year following diagnosis. These findings highlight the need for early recognition and multidisciplinary management of ICI-related cardiotoxicity.
Immune checkpoint inhibitors (ICIs) are central to modern cancer immunotherapy but may lead to cardiac immune-related adverse events (irAEs), including myocarditis, pericarditis, pericardial effusion, and tamponade. These complications, while rare, may significantly impact survival, yet contemporary real-world data on outcomes are limited.
Hypothesis:
Patients who develop cardiac irAEs after ICI initiation have worse one-year survival than those who do not.
Methods:
We performed a retrospective cohort study using the TriNetX US Collaborative Network, a federated database of electronic health records from multiple healthcare organizations (HCOs). We queried data from 57 HCOs. Adult cancer patients (≥18 years) receiving any ICI between January 1st, 2012 and December 31st, 2023 were included. Two cohorts were defined: those who developed cardiac irAEs and those who did not. Cardiac irAEs were identified using ICD-10-CM codes for myocarditis, pericarditis, pericardial effusion, and tamponade. Propensity score matching (1:1) was applied based on age, sex, race/ethnicity, cancer type, cardiovascular risk factors, and baseline comorbidities. The primary outcome was one-year all-cause mortality, assessed using Kaplan-Meier analysis.
Results:
Of 109,392 eligible patients, 6,925 (6.3%) developed cardiac irAEs and 102,467 did not. After matching and excluding those who died before the follow up period, 6,610 patients remained in the irAE cohort and 6,704 in the control group. The matched population had a mean age of 68.7 ± 12.7 years; 51.6% were male, 74.2% White, 11.3% Black, 4.4% Asian, and 5.2% Hispanic/Latino. Cardiovascular and oncologic comorbidities were well balanced. One-year mortality was significantly higher among those with cardiac irAEs (3,029/6,610; 45.8%) vs. controls (2,437/6,704; 36.4%), with a risk difference of 9.5% (95% CI: 7.8–11.1; p < 0.001). One-year survival probability was 50.11% in the irAE group vs. 61.51% in controls. The hazard ratio for death was 1.54 (95% CI: 1.46–1.62; p < 0.001).
Conclusion:
Cardiac irAEs following ICI therapy are linked to significantly worse one-year survival, with a 54% higher risk of death at any given time in the year following diagnosis. These findings highlight the need for early recognition and multidisciplinary management of ICI-related cardiotoxicity.
More abstracts on this topic:
Achieving Guidelines within a 24-Hour Movement Paradigm and Risk of Cardiovascular Disease and All-Cause Mortality in United States Adults
Boudreaux Benjamin, Xu Chang, Dooley Erin, Hornikel Bjoern, Munson Alexandra, Shechter Ari, Palta Priya, Gabriel Kelley, Diaz Keith
A Bleeding Heart: Chronic Pericarditis Manifesting as Recurrent Hemorrhagic Pericardial Effusion - Diagnostic Considerations with a PFO Closure Device and the Role of CT ImagingPatel Zeel, Liu Yang, Wengrofsky Perry, Yoon Sung-han