Final ID: MP794
Effects of Vutrisiran on Measures of Cardiac Structure, Function and Amyloid Burden by Cardiovascular Magnetic Resonance from the HELIOS-B Trial
Purpose: To analyze the impact of vutrisiran vs placebo on cardiac structure, function and amyloid burden in HELIOS-B participants.
Methods: In HELIOS-B ATTR-CM patients were randomized 1:1 to vutrisiran or placebo. The study population comprised HELIOS-B participants who underwent serial CMR assessments at the National Amyloidosis Centre as part of routine clinical care. CMRs were conducted at baseline, 1, 2, and 3-year timepoints during the HELIOS-B study. Image analysis was blinded to treatment allocation. Amyloid regression and progression were defined as an absolute reduction and increase in ECV of >5%, respectively. Changes in CMR parameters between baseline and follow-up were evaluated, and linear regression modelling was used to determine treatment effects.
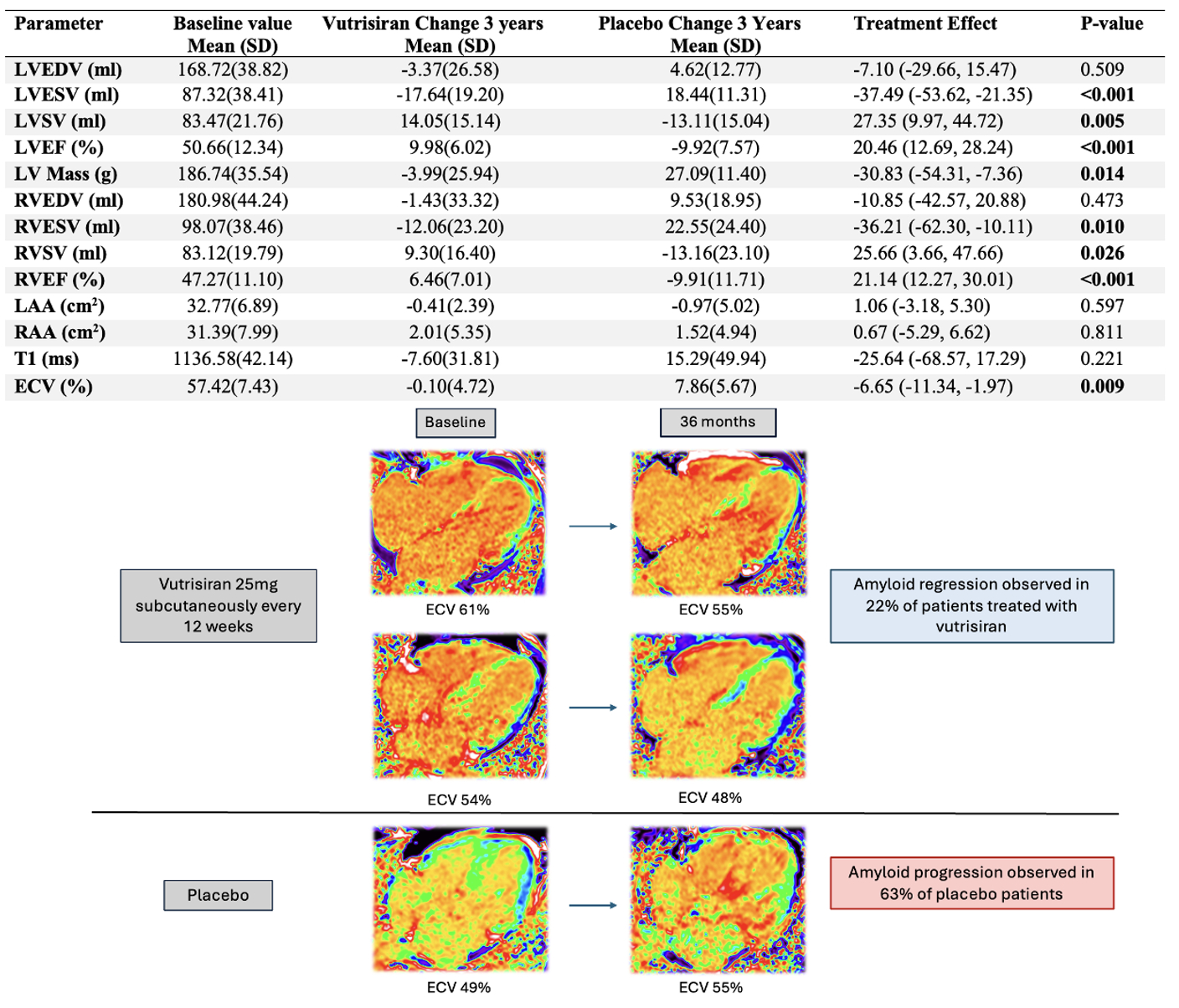
Results: Forty-three patients (mean (SD) age 75.0(5.67) years, 41/43male) underwent baseline CMR (21vutrisiran, 22placebo). Thirty-nine (21 vutrisiran, 18 placebo), 26 (14 vutrisiran, 12 placebo) and 17 (9 vutrisiran, 8 placebo) patients underwent 1, 2 and 3-year CMR, respectively. No patients received background tafamidis treatment. Baseline CMR characteristics were comparable between the treatment groups. At 3 years, vutrisiran led to significant increases in left and right ventricular ejection fractions (least square mean difference LVEF+20.5%, RVEF+21.1%; both p < 0.001), stroke volumes (LVSV+27.4 mL, RVSV+25.7 mL; p = 0.005 and p = 0.026, respectively), significant reductions in LVmass (–30.8 g; p = 0.014), and cardiac amyloid load (ECV –6.7%; p = 0.009) compared to placebo. For all parameters noted above, vutrisiran led to improvements while placebo led to deterioration. Amyloid regression was observed in 22% of vutrisiran patients; no placebo patients regressed. Conversely, 63% of placebo patients demonstrated progression vs 11% for vutrisiran. Significant changes in cardiac structure and function were emergent as early as year two.
Conclusion: In patients with ATTR-CM, treatment with vutrisiran was associated with improvements in cardiac structure, function and amyloid burden compared to placebo.
- Razvi, Yousuf ( University College London , London , United Kingdom )
- Bansilal, Sameer ( Alnlyam Pharmaceuticals , Cambridge , Massachusetts , United States )
- Eraly, Satish ( Alnlyam Pharmaceuticals , Cambridge , Massachusetts , United States )
- Aldinc, Emre ( Alnlyam Pharmaceuticals , Cambridge , Massachusetts , United States )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Gillmore, Julian ( University College London , London , United Kingdom )
- Fontana, Marianna ( University College London , London , United Kingdom )
- Sheikh, Awais ( University College London , London , United Kingdom )
- Patel, Rishi ( University College London , London , United Kingdom )
- Achten, Anouk ( Maastricht University CARIM School , Maastricht , Netherlands )
- Mansell, Josephine ( University College London , London , United Kingdom )
- Martinez-naharro, Ana ( University College London , London , United Kingdom )
- Venneri, Lucia ( University College London , London , United Kingdom )
- Hawkins, Philip ( University College London , London , United Kingdom )
- Vest, John ( Alnlyam Pharmaceuticals , Cambridge , Massachusetts , United States )
Meeting Info:
Session Info:
Under the Sheets: Contemporary Cardiac Amyloidosis Research
Saturday, 11/08/2025 , 09:15AM - 10:25AM
Moderated Digital Poster Session
More abstracts on this topic:
Quadri Fayz, Qazi Mariam, Teague Taylor
A Case of Caseous Mitral Annular Calcification and the Utility of Multimodality Cardiac ImagingNguyen Amanda, English Carter, Ghasemiesfe Ahmadreza, Venugopal Sandhya
More abstracts from these authors:
Porcari Aldostefano, Solomon Scott, Martinez-naharro Ana, Hawkins Philip, Gillmore Julian, Fontana Marianna, Dal Passo Beatrice, Venneri Lucia, Bandera Francesco, Razvi Yousuf, Sheikh Awais, Mansell Josephine, Zaro Elisa, Giampieri Gaia
Mapping the Myocardial Amyloid Burden in Transthyretin Cardiomyopathy: A Large-Scale Study Using Cardiac Magnetic Resonance and Extracellular Volume in 1,400 PatientsSheikh Awais, Knight Daniel, Virsinskaite Ruta, Kotecha Tushar, Lachmann Helen, Wechalekar Ashutosh, Gillmore Julian, Fontana Marianna, Achten Anouk, Razvi Yousuf, Porcari Aldostefano, Mansell Josephine, Venneri Lucia, Martinez-naharro Ana, Whelan Carol, Hawkins Philip