Final ID: MP2197
A Platelet Transcriptomic Signature Predicts Cardiovascular Risk in Peripheral Artery Disease
Abstract Body (Do not enter title and authors here): Background: Patients with peripheral artery disease have a markedly increased risk of cardiovascular morbidity and mortality. While platelets are key mediators in the pathogenesis of peripheral artery disease (PAD), few studies have characterized the PAD platelet transcriptome. The platelet transcriptome offers unique insights into both hemostatic, thrombotic, and inflammatory processes that may inform risk stratification in PAD patients.
Hypothesis: We hypothesized that platelet gene expression patterns would uncover distinct transcriptomic signatures associated with PAD prevalence, severity and clinical outcomes.
Methods: Participants with symptomatic PAD (n=171) were enrolled and had platelets collected prior to lower extremity revascularization (LER). RNA was extracted from isolated platelets and sequenced. Transcriptomic analysis identified signatures linked to PAD prevalence, severity, and cardiovascular events. Participants were followed for major adverse cardiovascular and limb events (MACLE, composite of death, MI, stroke, and major amputation). Statistical models were used to assess the association between platelet transcriptomics and clinical outcomes, with adjustments for relevant covariates.
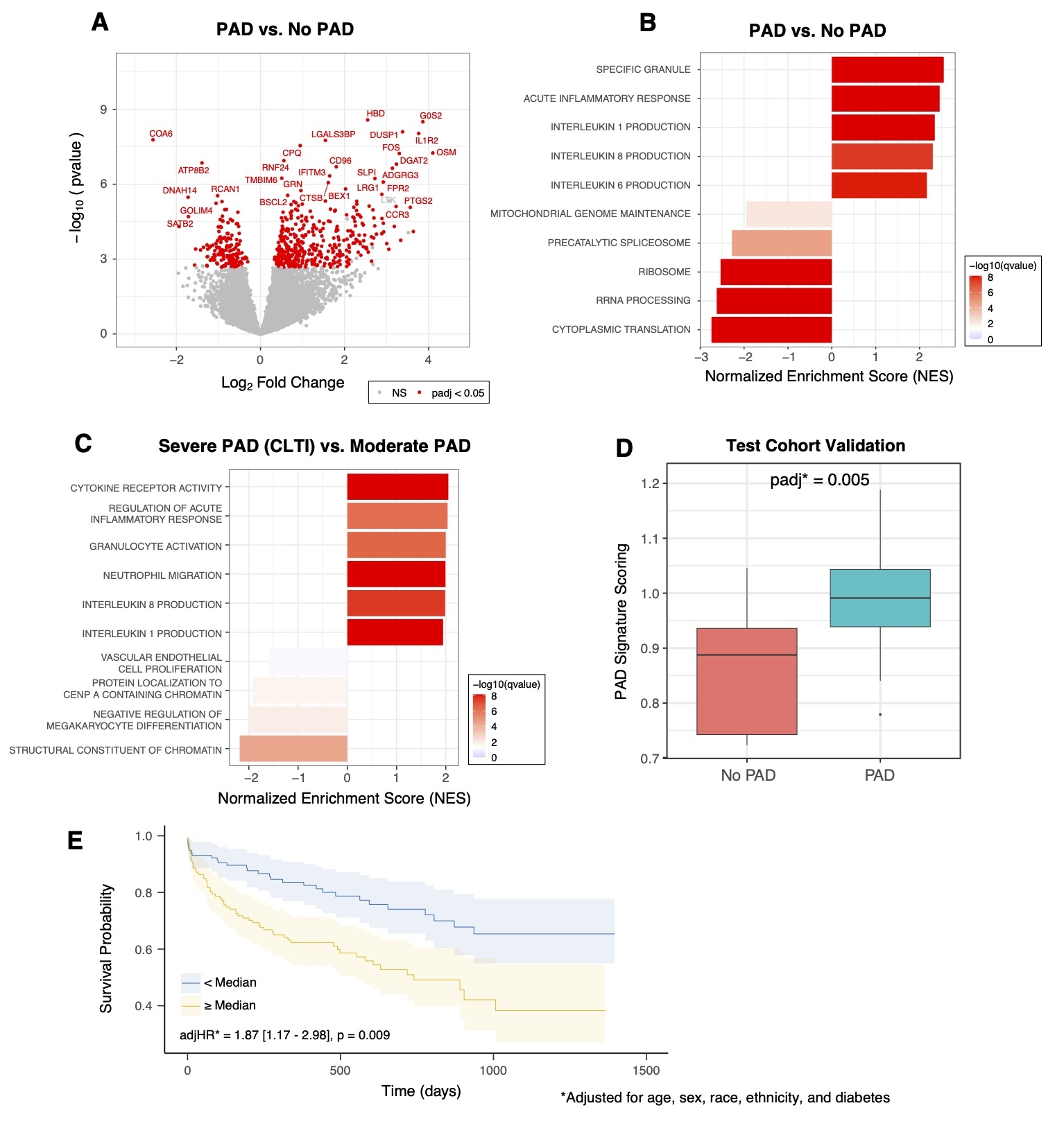
Results: Overall, mean age was 70 ± 10 years, 35% were female, 33% were non-White, 19% were Hispanic/Latino, and 50% had diabetes. In PAD patients compared to controls, 556 genes were identified as differentially expressed (padj<0.05), including 394 upregulated and 162 downregulated genes (Figure 1a). Pathway analysis revealed enrichment in inflammatory responses and granule release pathways (Figure 1b). Among PAD patients, those with chronic limb-threatening ischemia (CLTI) exhibited the highest enrichment in thrombotic and inflammatory pathways (Figure 1c). A 75-gene transcriptomic signature for PAD was developed and validated, demonstrating a significant association with PAD in a separate test cohort (padj=.005; Figure 1d). Finally, individuals with a score above the median demonstrated a higher risk for MACLE after multivariable adjustment (adjHR=1.87 [1.17-2.98], p=0.009; Figure 1e).
Conclusions: The platelet transcriptome in PAD patients reveals significant enrichment of pathways linked to granule release and inflammatory responses. A unique transcriptomic signature is strongly associated with PAD prevalence, severity, and cardiovascular outcomes, highlighting its potential as a novel biomarker for risk stratification in patients undergoing LER.
Hypothesis: We hypothesized that platelet gene expression patterns would uncover distinct transcriptomic signatures associated with PAD prevalence, severity and clinical outcomes.
Methods: Participants with symptomatic PAD (n=171) were enrolled and had platelets collected prior to lower extremity revascularization (LER). RNA was extracted from isolated platelets and sequenced. Transcriptomic analysis identified signatures linked to PAD prevalence, severity, and cardiovascular events. Participants were followed for major adverse cardiovascular and limb events (MACLE, composite of death, MI, stroke, and major amputation). Statistical models were used to assess the association between platelet transcriptomics and clinical outcomes, with adjustments for relevant covariates.
Results: Overall, mean age was 70 ± 10 years, 35% were female, 33% were non-White, 19% were Hispanic/Latino, and 50% had diabetes. In PAD patients compared to controls, 556 genes were identified as differentially expressed (padj<0.05), including 394 upregulated and 162 downregulated genes (Figure 1a). Pathway analysis revealed enrichment in inflammatory responses and granule release pathways (Figure 1b). Among PAD patients, those with chronic limb-threatening ischemia (CLTI) exhibited the highest enrichment in thrombotic and inflammatory pathways (Figure 1c). A 75-gene transcriptomic signature for PAD was developed and validated, demonstrating a significant association with PAD in a separate test cohort (padj=.005; Figure 1d). Finally, individuals with a score above the median demonstrated a higher risk for MACLE after multivariable adjustment (adjHR=1.87 [1.17-2.98], p=0.009; Figure 1e).
Conclusions: The platelet transcriptome in PAD patients reveals significant enrichment of pathways linked to granule release and inflammatory responses. A unique transcriptomic signature is strongly associated with PAD prevalence, severity, and cardiovascular outcomes, highlighting its potential as a novel biomarker for risk stratification in patients undergoing LER.
More abstracts on this topic:
Age-linked PVAT dysfunction and sex-specific signatures: Unveiling the pathobiology and phenotypes associated with CABG graft patency
Ryu Ji-yeon, Jang Eui Hwa, Shin Yejin, Youn Young-nam
The Platelet Reactivity ExpreSsion Score and Response to Antiplatelet TherapyDiblasio Rebecca, Muller Matthew, Ruggles Kelly, Voora Deepak, Barrett Tessa, Berger Jeffrey