Final ID: Su4070
Assessing Mitochondrial Viability Under High-Calcium Stress: Relevance to Intracoronary Therapeutics
Abstract Body (Do not enter title and authors here): Background: Extracellular calcium above basal cytosolic levels disrupts mitochondrial homeostasis, causing swelling, inner membrane breakdown, loss of membrane potential, and reduced ATP production. These effects challenge mitochondrial transplantation—especially with intracoronary delivery in calcium-rich environments. Though promising, the infusion approach remains controversial. Some studies report rapid mitochondrial damage in physiological calcium, raising concerns about membrane rupture and release of pro-apoptotic or inflammatory contents. Others report survival and uptake, suggesting a viable mitochondrial subpopulation may persist. Resolving these discrepancies is key for clinical translation.
Research Question: How do physiologically relevant extracellular calcium concentrations and exposure durations affect mitochondrial viability and membrane integrity under transplantation-like conditions?
Methods: Mitochondria were isolated from mammalian cells via differential centrifugation. Some were freeze-thawed to model membrane damage. Samples were incubated in calcium-free media with 0, 0.65-, 1.3-, or 2.6-mM Ca2+. Membrane potential was measured using MitoTracker Red FM fluorescence (mean fluorescence intensity, MFI). Impedance-based Coulter counter analysis evaluated membrane integrity, with signal loss indicating lysis or swelling.
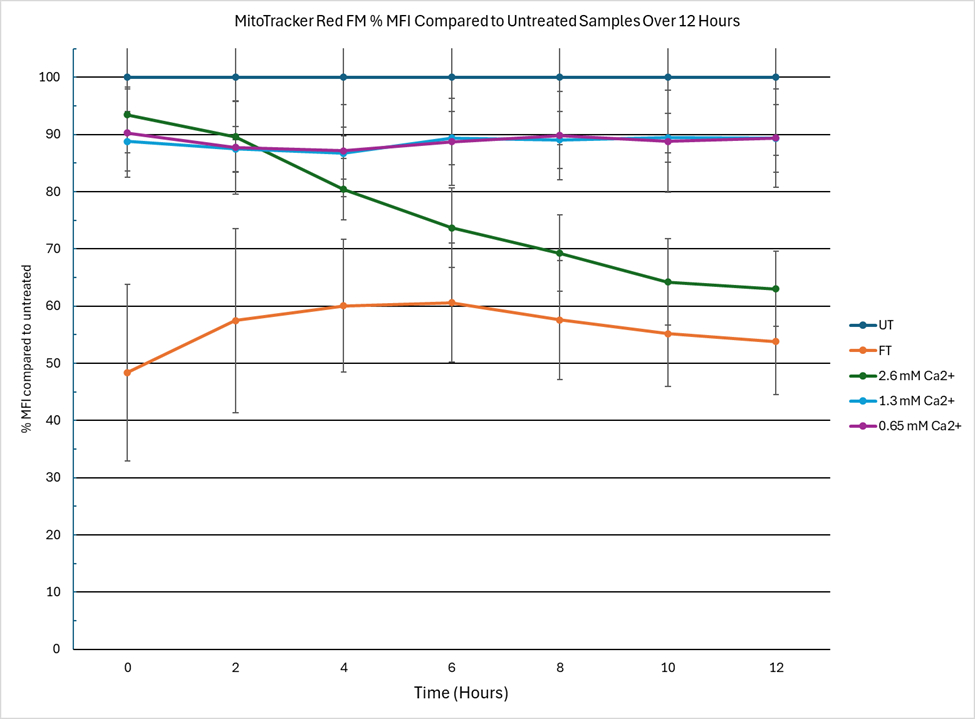
Results: Calcium exposure caused time- and dose-dependent mitochondrial damage. At 2 hours, all calcium-treated groups showed a 10–20% MFI decrease (p < 0.01), while freeze-thawed controls showed ~50% loss. The 2.6 mM calcium group showed irreversible decline similar to freeze-thawed samples. In contrast, 0.65- and 1.3-mM groups partially recovered, retaining ~90% MFI at 12 hours. Early MFI drops in these groups were transient. Coulter counter data followed similar trends but showed more severe damage: by 12 hours, 2.6 mM and freeze-thawed groups dropped to ~20%, while 1.3 and 0.65 mM groups retained ~40% and ~60%, respectively.
Conclusion: Extracellular calcium causes progressive mitochondrial damage, but within the first 2 hours—when uptake is most likely—up to 90% of mitochondria remained viable at 1.3 mM Ca2+, the physiological level. These results support the feasibility of early transplantation. Coulter counter data validate a label-free method to assess viability. Together, these findings guide safe and effective use of mitochondrial therapies in calcium-rich environments.
Research Question: How do physiologically relevant extracellular calcium concentrations and exposure durations affect mitochondrial viability and membrane integrity under transplantation-like conditions?
Methods: Mitochondria were isolated from mammalian cells via differential centrifugation. Some were freeze-thawed to model membrane damage. Samples were incubated in calcium-free media with 0, 0.65-, 1.3-, or 2.6-mM Ca2+. Membrane potential was measured using MitoTracker Red FM fluorescence (mean fluorescence intensity, MFI). Impedance-based Coulter counter analysis evaluated membrane integrity, with signal loss indicating lysis or swelling.
Results: Calcium exposure caused time- and dose-dependent mitochondrial damage. At 2 hours, all calcium-treated groups showed a 10–20% MFI decrease (p < 0.01), while freeze-thawed controls showed ~50% loss. The 2.6 mM calcium group showed irreversible decline similar to freeze-thawed samples. In contrast, 0.65- and 1.3-mM groups partially recovered, retaining ~90% MFI at 12 hours. Early MFI drops in these groups were transient. Coulter counter data followed similar trends but showed more severe damage: by 12 hours, 2.6 mM and freeze-thawed groups dropped to ~20%, while 1.3 and 0.65 mM groups retained ~40% and ~60%, respectively.
Conclusion: Extracellular calcium causes progressive mitochondrial damage, but within the first 2 hours—when uptake is most likely—up to 90% of mitochondria remained viable at 1.3 mM Ca2+, the physiological level. These results support the feasibility of early transplantation. Coulter counter data validate a label-free method to assess viability. Together, these findings guide safe and effective use of mitochondrial therapies in calcium-rich environments.
More abstracts on this topic:
Cardiac Mitochondrial Dysfunction Impairs Mitochondrial Complex Activities And Induces UPRmt Stress Responses In The Cortex And Hippocampus
Muthu Sakthijothi, Tran Zinnia, Karelina Kate, Velayutham Murugesan, Meadows Ethan, Hollander John, Sundararajan Venkatesh
Acetylation of Mitochondrial Cyclophilin D Increases vascular Oxidative Stress, Induces Glycolitic Switch, Promotes Endothelial Dysfunction and HypertensionDikalov Sergey, Sack Michael, Dikalova Anna, Fehrenbach Daniel, Mayorov Vladimir, Panov Alexander, Ao Mingfang, Lantier Louise, Amarnath Venkataraman, Lopez Marcos, Billings Frederic