Final ID: MP1703
Two Mechanisms, One Valve: A Structural and Hemodynamic Comparison of Rheumatic and MAC-Induced Mitral Stenosis
Abstract Body (Do not enter title and authors here): Background: Mitral annular calcification (MAC) and rheumatic heart disease represent two distinct mechanisms leading to mitral stenosis (MS). MAC is a common degenerative process associated with increased cardiovascular risk, often resulting in anatomically and hemodynamically unique forms of MS. In contrast, rheumatic mitral stenosis (RMS) arises from inflammatory scarring and commissural fusion. These differing pathophysiologies produce distinct structural and flow characteristics that challenge conventional diagnostic and therapeutic strategies. Understanding these contrasts is essential for accurate assessment and tailored management of MS.
Research Questions/Hypothesis: This study aimed to 1) compare structural and flow characteristics, including kinetic energy losses, among MAC-related MS, rheumatic MS (RMS), and normal mitral valves (NMV), and 2) assess the relevance of conventional diagnostic metrics in MAC. We hypothesized that MAC-related MS has distinct structural and hemodynamic features compared to RMS and NMV.
Methods: We analyzed 3D transesophageal echocardiographic data from 70 patients (22 NMV, 26 RMS, 22 MAC). Linear, area, and volumetric measurements characterized each valve type. Representative valves were 3D-printed in silicone for in vitro testing in a heart flow simulator. Transmitral flow was visualized via Particle Image Velocimetry (PIV), and flow energetics were quantified. Coefficients of contraction (CoC) were calculated from geometric and effective orifice areas.
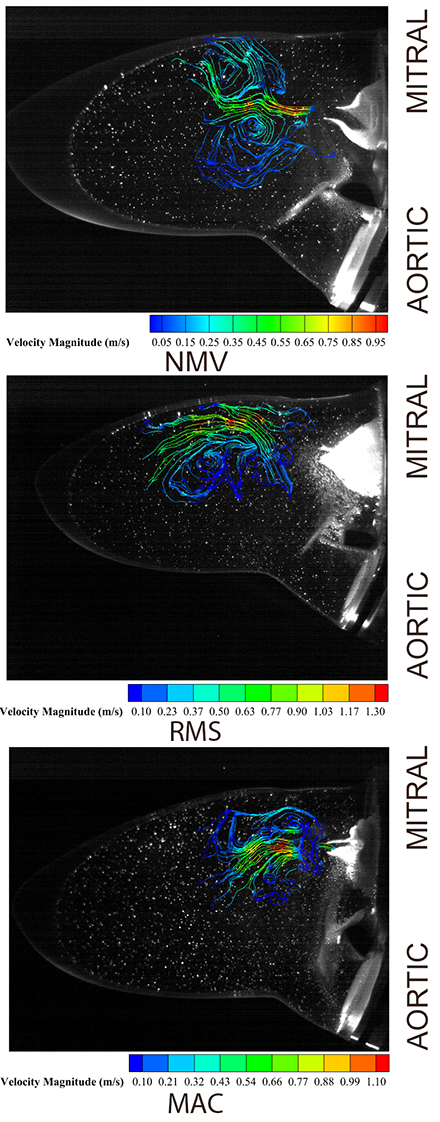
Results: Compared to RMS, MAC MS had smaller antero-posterior annulus dimensions, reduced valve volume, and lower CoC. MAC showed the highest transmitral velocities and energy loss in vitro. Neither MAC nor RMS models formed transmitral vortex rings, unlike the normal model. Despite a larger geometric orifice, MAC caused a greater pressure drop than RMS, likely due to disrupted flow and lower CoC. These changes in geometry and flow may affect left ventricular function.
Conclusions: The lack of transmitral vortex rings in MAC and RMS, combined with higher velocities and energy loss in MAC, reflects altered flow dynamics versus normal valves. These results highlight the need for disease-specific diagnostics and multimodal imaging to improve clinical decisions and guide therapies. Limitations include 2D velocity measurements and reliance on three valve models. Future work should explore more valve types and conditions.
Research Questions/Hypothesis: This study aimed to 1) compare structural and flow characteristics, including kinetic energy losses, among MAC-related MS, rheumatic MS (RMS), and normal mitral valves (NMV), and 2) assess the relevance of conventional diagnostic metrics in MAC. We hypothesized that MAC-related MS has distinct structural and hemodynamic features compared to RMS and NMV.
Methods: We analyzed 3D transesophageal echocardiographic data from 70 patients (22 NMV, 26 RMS, 22 MAC). Linear, area, and volumetric measurements characterized each valve type. Representative valves were 3D-printed in silicone for in vitro testing in a heart flow simulator. Transmitral flow was visualized via Particle Image Velocimetry (PIV), and flow energetics were quantified. Coefficients of contraction (CoC) were calculated from geometric and effective orifice areas.
Results: Compared to RMS, MAC MS had smaller antero-posterior annulus dimensions, reduced valve volume, and lower CoC. MAC showed the highest transmitral velocities and energy loss in vitro. Neither MAC nor RMS models formed transmitral vortex rings, unlike the normal model. Despite a larger geometric orifice, MAC caused a greater pressure drop than RMS, likely due to disrupted flow and lower CoC. These changes in geometry and flow may affect left ventricular function.
Conclusions: The lack of transmitral vortex rings in MAC and RMS, combined with higher velocities and energy loss in MAC, reflects altered flow dynamics versus normal valves. These results highlight the need for disease-specific diagnostics and multimodal imaging to improve clinical decisions and guide therapies. Limitations include 2D velocity measurements and reliance on three valve models. Future work should explore more valve types and conditions.
More abstracts on this topic:
A Deep Learning Digital Biomarker for Mitral Valve Prolapse using Echocardiogram Videos
Al-alusi Mostafa, Khurshid Shaan, Sanborn Danita, Picard Michael, Ho Jennifer, Maddah Mahnaz, Ellinor Patrick, Lau Emily, Small Aeron, Reeder Christopher, Shnitzer Dery Tal, Andrews Carl, Kany Shinwan, Ramo Joel, Haimovich Julian
A Comparison Between Global Longitudinal Strain (GLS) Derived with CMR Feature-Tracking (CMR-FT) and 2D Speckle-Tracking Echocardiography (2D-STE) to Monitor Cancer Therapy-Related Cardiac Dysfunction (CTRCD)Kar Julia, Cohen Michael, Revere Cherie, Mcquiston Samuel, Malozzi Christopher