Final ID: MP1724
Walking Impairment Questionnaire score declines after lower extremity revascularization are linked to adverse cardiovascular events and rivaroxaban dose not affect walking ability
Abstract Body (Do not enter title and authors here): Backgrounds: The Walking Impairment Questionnaire (WIQ) is a validated tool for assessing patient reported outcomes related to walking in patients with peripheral artery disease (PAD). The clinical outcomes associated with WIQ changes after successful lower extremity revascularization (LER) over time are not well described.
Research Questions: We aimed to evaluate the outcomes associated with change in WIQ score after LER and whether WIQ was effected by rivaroxaban.
Methods: This study was conducted on 4,448 participants of the VOYAGER PAD trial who had WIQ data available at both 1 and 12 months. Individuals who experienced unplanned LER, major amputation, or acute limb ischemia (ALI) during this period were excluded. The difference in total WIQ scores between the 1- and 12-month time points was calculated, and the degree of change was classified into 5 categories using 5-point increments. Total events of the primary efficacy outcome—a composite of ALI, major amputation for vascular causes, myocardial infarction, ischemic stroke, or cardiovascular death—occurring beyond 12 months were evaluated across categories of total WIQ score changes. The change in WIQ scores was compared between the rivaroxaban and placebo groups.
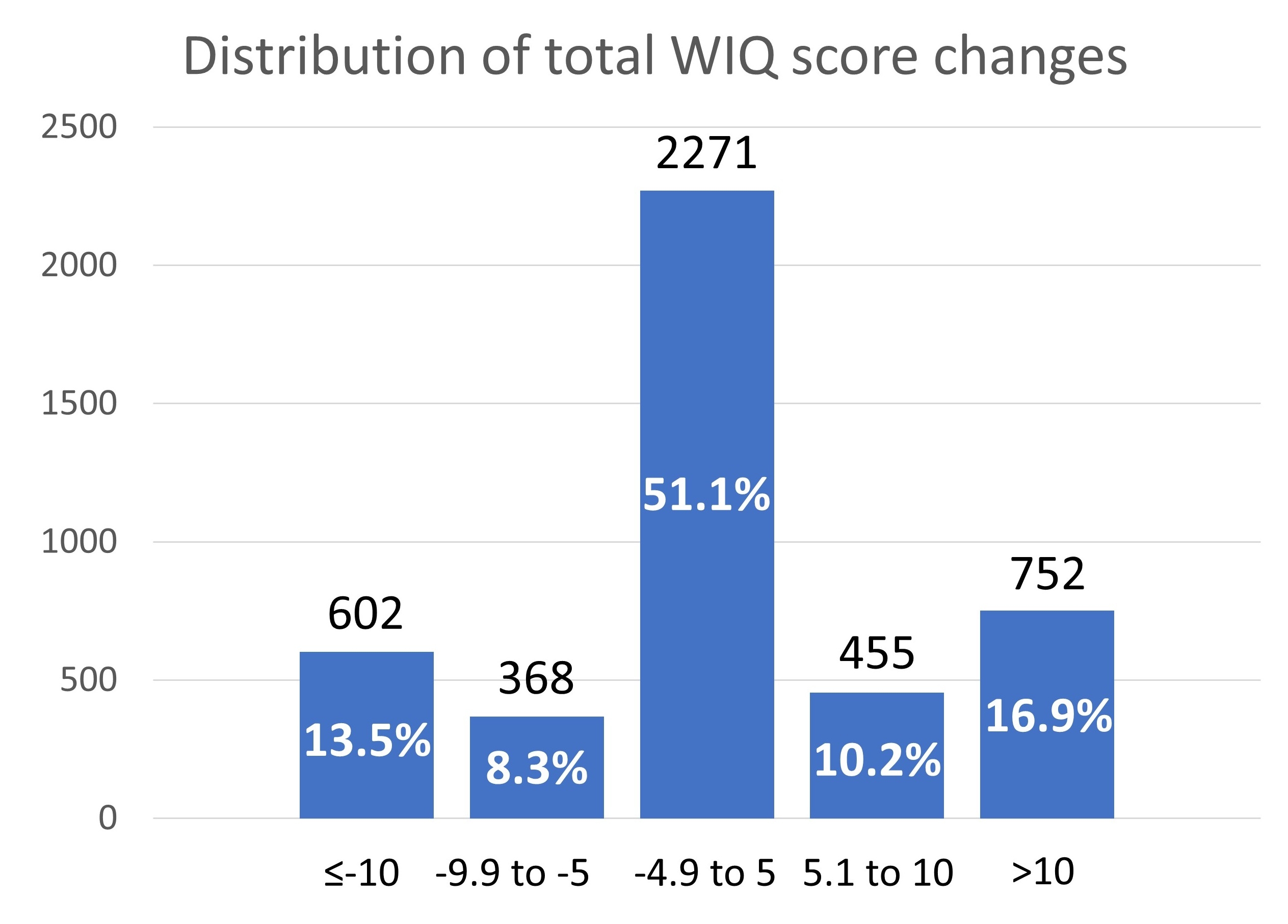
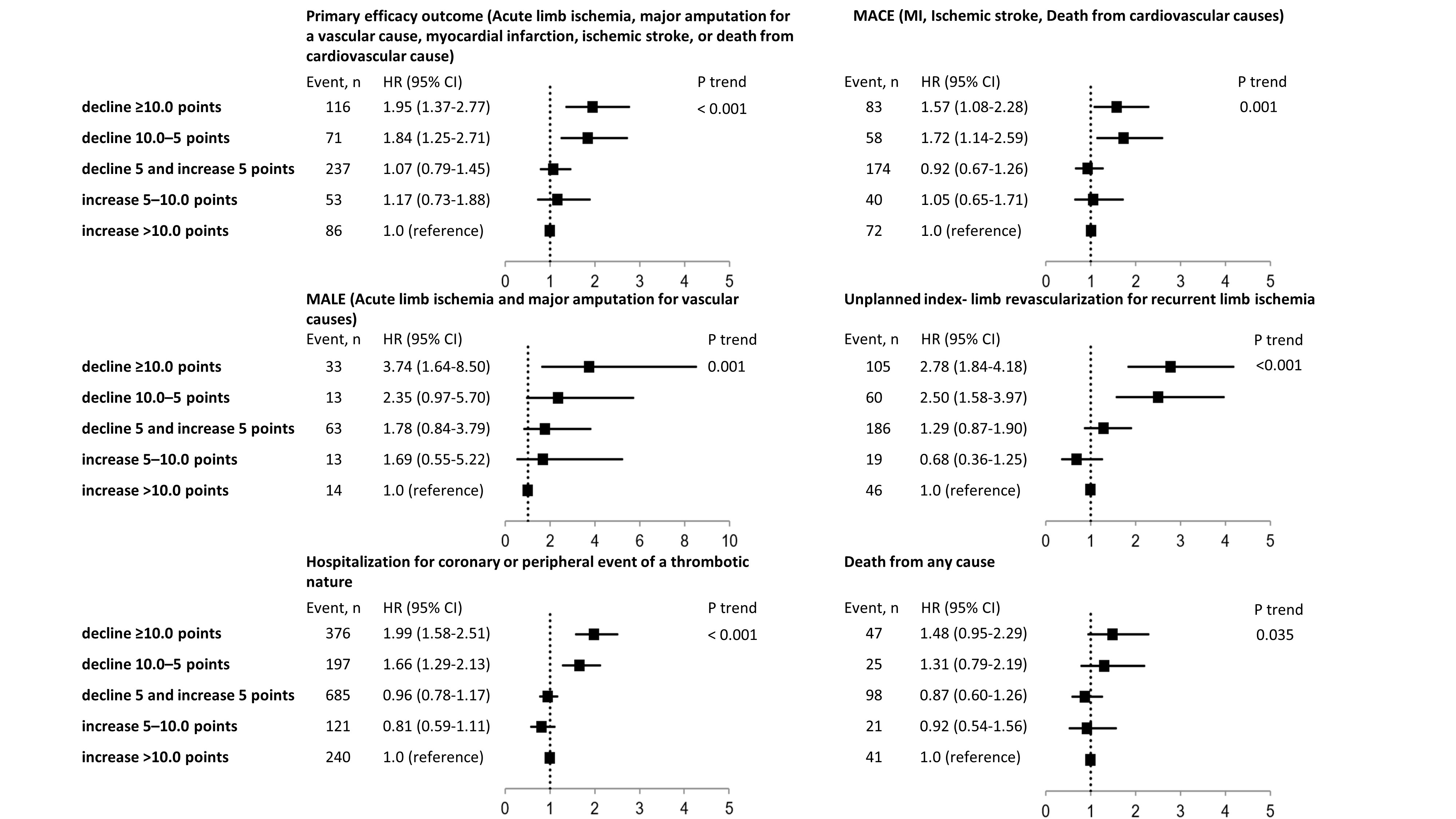
Results: After LER, 13.5% exhibited total WIQ score worsening more than 10 points during the 12-month follow-up period, while 16.9% showed an improvement of ≥10 points (Figure 1). Patients who experienced ≥10 points decline in the total WIQ score between 1 and 12 months had a higher rate of primary efficacy outcome (hazard ratio [HR]: 1.95 [1.37–2.77]), major adverse cardiovascular events (HR: 1.57 [1.08–2.28]), major adverse limb events (HR: 3.74 [1.64–8.50]), unplanned revascularization (HR: 2.78 [1.84–4.18]), and coronary artery disease(CAD)/PAD hospitalization (HR: 1.99 [1.58–2.51]) compared with those who improved WIQ score (Figure 2). The independent factors associated with a decline of ≥10 points in WIQ score included older age, current smoking, symptomatic CAD, and endovascular treatment. There was no difference in WIQ score changes between rivaroxaban and placebo groups (Figure 2).
Conclusion: In this post-hoc analysis of VOYAGER PAD trial, patients who experienced ≥10 points decline in total WIQ score over the 12-month follow-up after LER were at a greater risk of adverse cardiovascular and limb events compared with those who improved WIQ score. There was no association between treatment with rivaroxaban and WIQ score changes.
Research Questions: We aimed to evaluate the outcomes associated with change in WIQ score after LER and whether WIQ was effected by rivaroxaban.
Methods: This study was conducted on 4,448 participants of the VOYAGER PAD trial who had WIQ data available at both 1 and 12 months. Individuals who experienced unplanned LER, major amputation, or acute limb ischemia (ALI) during this period were excluded. The difference in total WIQ scores between the 1- and 12-month time points was calculated, and the degree of change was classified into 5 categories using 5-point increments. Total events of the primary efficacy outcome—a composite of ALI, major amputation for vascular causes, myocardial infarction, ischemic stroke, or cardiovascular death—occurring beyond 12 months were evaluated across categories of total WIQ score changes. The change in WIQ scores was compared between the rivaroxaban and placebo groups.
Results: After LER, 13.5% exhibited total WIQ score worsening more than 10 points during the 12-month follow-up period, while 16.9% showed an improvement of ≥10 points (Figure 1). Patients who experienced ≥10 points decline in the total WIQ score between 1 and 12 months had a higher rate of primary efficacy outcome (hazard ratio [HR]: 1.95 [1.37–2.77]), major adverse cardiovascular events (HR: 1.57 [1.08–2.28]), major adverse limb events (HR: 3.74 [1.64–8.50]), unplanned revascularization (HR: 2.78 [1.84–4.18]), and coronary artery disease(CAD)/PAD hospitalization (HR: 1.99 [1.58–2.51]) compared with those who improved WIQ score (Figure 2). The independent factors associated with a decline of ≥10 points in WIQ score included older age, current smoking, symptomatic CAD, and endovascular treatment. There was no difference in WIQ score changes between rivaroxaban and placebo groups (Figure 2).
Conclusion: In this post-hoc analysis of VOYAGER PAD trial, patients who experienced ≥10 points decline in total WIQ score over the 12-month follow-up after LER were at a greater risk of adverse cardiovascular and limb events compared with those who improved WIQ score. There was no association between treatment with rivaroxaban and WIQ score changes.
More abstracts on this topic:
Bleeding with the FXI Inhibitor Abelacimab compared with Rivaroxaban in Patients on Antiplatelet therapy: A Prespecified Analysis of the AZALEA-TIMI 71 Trial
Al Said Samer, Goodman Shaun, Joung Boyoung, Kiss Robert, Spinar Jindrich, Wojakowski Wojciech, Weitz Jeffrey, Bloomfield Dan, Sabatine Marc, Ruff Christian, Patel Siddharth, Giugliano Robert, Morrow David, Goodrich Erica, Murphy Sabina, Hug Bruce, Parker Sanobar, Chen Shih-ann
A Novel Retrieval-Augmented Generation Approach that Leverages Large Language Models to Parse EHR Data Enhances the Detection of Barriers in Guideline-Directed Medical Therapy Use in Heart FailureAdejumo Philip, Thangaraj Phyllis, Croon Philip, Dhingra Lovedeep, Aminorroaya Arya, Pedroso Aline, Khera Rohan