Final ID: MP331
Non-invasive Diastolic Stress Testing in a Large Observational Cohort Study: Insights from the Dallas Heart Study
Abstract Body (Do not enter title and authors here): Introduction: Despite its increasing prevalence and significant morbidity/mortality in the aging population, heart failure with preserved ejection fraction (HFpEF) remains challenging to diagnose as its symptoms may occur only during exercise. In smaller studies, non-invasive diastolic stress testing has been validated in diagnosing exercise-induced increase in diastolic filling pressures. However, its feasibility in a large real-world population has not been tested.
Aims: The Dallas Heart Study is a multiethnic, probability-based, population cohort study of Dallas County residents with deliberate oversampling of black individuals. The study sample consisted of 712 participants with LVEF≥50% and free of prevalent HF who attended the 3rd phase of the Dallas Heart Study (2021-2024) and underwent diastolic stress echocardiography.
Methods: Participants underwent comprehensive resting echo followed by a submaximal stress echo protocol which includes pedaling on a supine echocardiography bicycle at a fixed workload of 30W. Per 2016 stress echocardiography recommendations by the American Society of Echocardiography (ASE), a definitive abnormal test was defined when the following criteria are met: septal E/e′ ratio>15, average E/e′>14, peak TR velocity>2.8 m/s with exercise, and either septal e′<7 or lateral e′<10 cm/s at rest.
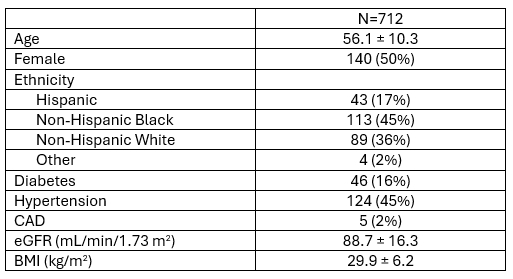
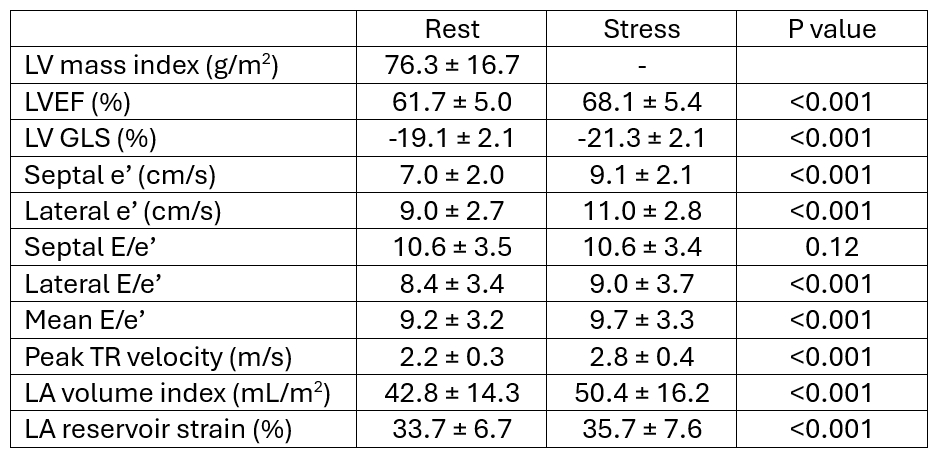
Results: Mean age was 59±11 years, and 56% were women (Table 1). 48% of the participants were Black, 32% White, and 18% Hispanic. Mean BMI was 31±7 kg/m2, 23% had diabetes, and 59% had hypertension. Between rest and stress (Table 2), there were statistically significant increases in LVEF, LV GLS, e’, mean E/e’, peak TR velocity, LA volume index, and LA reservoir strain. At rest, 5% of the participants had abnormal E/e’ (defined as >14), while at stress 8% did. To identify predictors of abnormal E/e’, backwards (P <0.001) multivariable stepwise regression modeling was performed on baseline demographics and resting echo variables and found lateral E/e’ as the only independent predictor (OR 1.86, 95% CI 1.40-2.46). Notably, 2% of the participants were found to have a definite abnormal diastolic stress test per ASE recommendation.
Conclusion: We have demonstrated the feasibility of performing non-invasive diastolic stress testing on a large contemporary real-world cohort. With rapidly emerging HFpEF therapies, more widespread adoption of an effective and safe diagnostic tool for HFpEF is needed to warrant adequate and individualized treatment.
Aims: The Dallas Heart Study is a multiethnic, probability-based, population cohort study of Dallas County residents with deliberate oversampling of black individuals. The study sample consisted of 712 participants with LVEF≥50% and free of prevalent HF who attended the 3rd phase of the Dallas Heart Study (2021-2024) and underwent diastolic stress echocardiography.
Methods: Participants underwent comprehensive resting echo followed by a submaximal stress echo protocol which includes pedaling on a supine echocardiography bicycle at a fixed workload of 30W. Per 2016 stress echocardiography recommendations by the American Society of Echocardiography (ASE), a definitive abnormal test was defined when the following criteria are met: septal E/e′ ratio>15, average E/e′>14, peak TR velocity>2.8 m/s with exercise, and either septal e′<7 or lateral e′<10 cm/s at rest.
Results: Mean age was 59±11 years, and 56% were women (Table 1). 48% of the participants were Black, 32% White, and 18% Hispanic. Mean BMI was 31±7 kg/m2, 23% had diabetes, and 59% had hypertension. Between rest and stress (Table 2), there were statistically significant increases in LVEF, LV GLS, e’, mean E/e’, peak TR velocity, LA volume index, and LA reservoir strain. At rest, 5% of the participants had abnormal E/e’ (defined as >14), while at stress 8% did. To identify predictors of abnormal E/e’, backwards (P <0.001) multivariable stepwise regression modeling was performed on baseline demographics and resting echo variables and found lateral E/e’ as the only independent predictor (OR 1.86, 95% CI 1.40-2.46). Notably, 2% of the participants were found to have a definite abnormal diastolic stress test per ASE recommendation.
Conclusion: We have demonstrated the feasibility of performing non-invasive diastolic stress testing on a large contemporary real-world cohort. With rapidly emerging HFpEF therapies, more widespread adoption of an effective and safe diagnostic tool for HFpEF is needed to warrant adequate and individualized treatment.
More abstracts on this topic:
A Bleeding Heart: Chronic Pericarditis Manifesting as Recurrent Hemorrhagic Pericardial Effusion - Diagnostic Considerations with a PFO Closure Device and the Role of CT Imaging
Patel Zeel, Liu Yang, Wengrofsky Perry, Yoon Sung-han
A Case Report: Outpatient Diagnosis of Venous Stent Migration - Avoiding Catastrophic OutcomesBasnyat Anouksha, Pamganamamula Madhu, Naidu Raja, Pamganamamula Teja, Manchiraju Srinidhi, Gaddam Srilakshmi, Panganamamula Lalitha