Final ID: MP2598
A Multicenter Study of Detection of Pulmonary Hypertension Based on Point-of-Care 12- Lead ECG Data
Hypothesis and Purpose: To evaluate the performance of a previously trained, ECG-AI algorithm to detect PH (ECG-AI PH) using real-world data (RWD) collected in a multicenter, validation study. A joint primary hypothesis required sensitivity (Sn), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) to exceed the null values of 76%, 70%, 10% and 90%.
Study Design and Methods: This retrospective validation study was conducted at 5 geographically diverse U.S. health systems. Adult subjects were eligible for inclusion if they had a 12-lead ECG paired with an echocardiogram (Echo) in which tricuspid regurgitation velocity (TRV) was recorded, following presentation with dyspnea. Patients were classified according to echocardiographic criteria as either PH (PH+, TRV >3.4 m/s) or controls (PH-, TRV ≤2.8 m/s) to simulate the real-world use of ECG-AI, where a positive result could lead to a follow-up Echo. The study database was locked before processing the digital ECGs with ECG-AI PH. Performance was also estimated in a subset of subjects that later had a right heart catheterization using mPAP ≥ 20mmHg as the definition of PH.
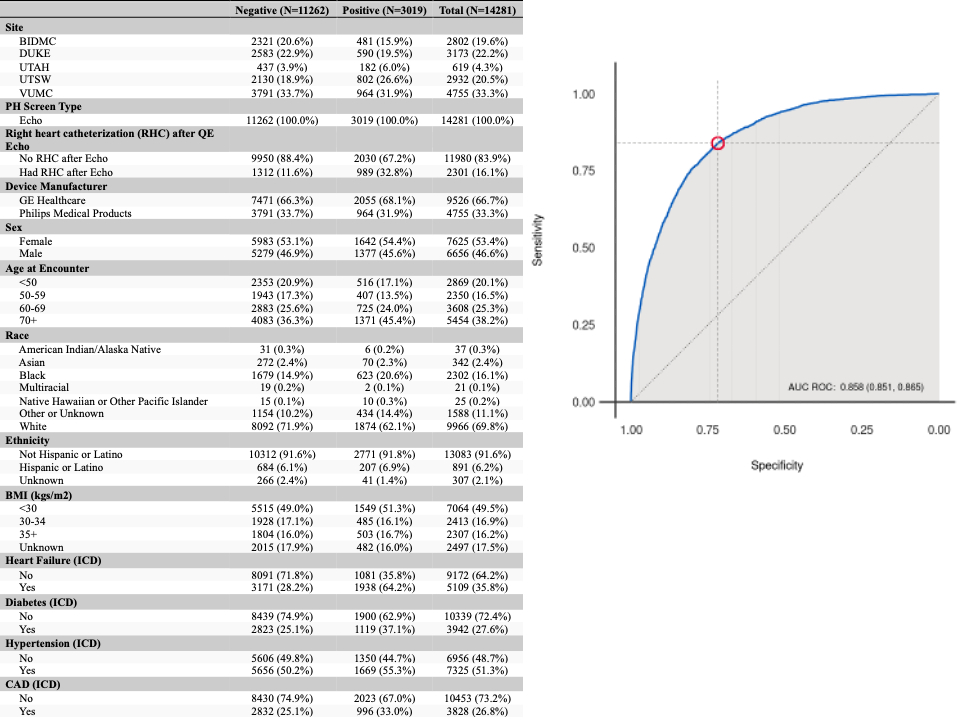
Results: A total of 14281 subjects (53% female, 63% aged 50+ years) met the inclusion criteria, including 3019 PH+ cases and 11262 PH- controls (Figure). Sn and Sp were 84.0% (95% CI: 82.6%, 85.3%) and 71.7% (95% CI: 70.9%, 72.6%), respectively. The positive and negative predictive values were 18.9% (95% CI: 18.4%, 19.4%) and 98.3% (95% CI: 98.1%, 98.4%), respectively, at 7.3% prevalence. Each endpoint met pre-defined performance criteria. In the subset of 1683 subjects with a follow up RHC, performance remained robust (Sn 85% (1155/1358); Sp 46% (151/325)).
Conclusion: While ECG-AI PH was first developed as an investigational tool to detect PH, continued development as software as a medical device for clinical use demonstrated that the algorithm retained strong performance to detect PH in diverse, non-overlapping clinical settings and patient populations.
- Dubrock, Hilary ( Mayo Clinic , Rochester , Minnesota , United States )
- Wieczorek, Mikolaj ( Mayo Clinic , Jacksonville , Florida , United States )
- Hackett, Sarah ( Anumana, Inc , Cambridge , Massachusetts , United States )
- Alger, Heather ( Anumana, Inc , Cambridge , Massachusetts , United States )
- Carlson, Katherine ( Anumana, Inc , Cambridge , Massachusetts , United States )
- Klugherz, Paul ( Mayo Clinic , Rochester , Minnesota , United States )
- Carter, Rickey ( Mayo Clinic , Jacksonville , Florida , United States )
- Wagner, Tyler ( Anumana, Inc , Cambridge , Massachusetts , United States )
- Johnson, Patrick ( Mayo Clinic , Rochester , Minnesota , United States )
- Frantz, Robert ( MAYO CLINIC , Rochester , Minnesota , United States )
- Strom, Jordan ( Harvard Medical School , Milton , Massachusetts , United States )
- Waks, Jonathan ( Beth Israel Deaconess Medical Cente , Newton Center , Massachusetts , United States )
- Agarwal, Richa ( Duke University , Durham , North Carolina , United States )
- Hemnes, Anna ( VANDERBILT UNIVERSITY , Nashville , Tennessee , United States )
- Steinberg, Benjamin ( University of Utah , Salt Lake City , Utah , United States )
- Pandey, Ambarish ( UT Southwestern Medical Center , Dallas , Texas , United States )
Meeting Info:
Session Info:
AI and Novel Biomarkers in PH: New Frontiers in Pulmonary Vascular Medicine
Monday, 11/10/2025 , 09:15AM - 09:55AM
Moderated Digital Poster Session
More abstracts on this topic:
Casula Manuela, Galimberti Federica, Olmastroni Elena, Arca Marcello, Averna Maurizio, Catapano Alberico
A Case Report of Cardiac Tamponade due to Mycoplasma Pneumoniae-induced Pericarditis - A Rare Complication of a Commonly seen Bacterial InfectionPatel Vidhi, Maharjan Reeju, Okan Tetyana, Singh Bhupinder, Colasacco Joseph
More abstracts from these authors:
Desai Akshay, Pandey Ambarish, Suratekar Rohit, Alger Heather, Awasthi Samir, Ahmad Faraz, Oh Jae, Khan Sadiya, Shah Sanjiv
Evaluation of a Novel Artificial Intelligence Electrocardiogram Tool for Early Identification of Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary HypertensionDubrock Hilary, Carlson Katherine, Alger Heather, Frantz Robert, Wagner Tyler