Final ID: Mo4002
Non Muscle Myosin IIC Density and Filament Length Increases in Neonatal Rat Ventricular Myocyte under Isoproterenol Stress
Abstract Body (Do not enter title and authors here): Background:
Myh14, encoding non-muscle myosin IIC (NMIIC), is a proposed negative regulator of isoproterenol (ISO)-induced cardiac hypertrophy. ISO upregulated mRNA transcript level of Myh14 in NRVM. In vivo, Myh14 knockout (KO) mice exhibit exaggerated ISO-induced left ventricular (LV) hypertrophy. In neonatal rat ventricular myocytes (NRVMs), Myh14 knockdown leads to poor adhesion and survival, but its protein localization and organization in cardiomyocytes remain unexplored. Based on findings in non-cardiac cells, MYH14 can organize into either filaments or puncta, with punctate separation potentially reflecting sarcomeric length. We aimed to define MYH14 subcellular organization and its changes under hypertrophic stress.
Methods:
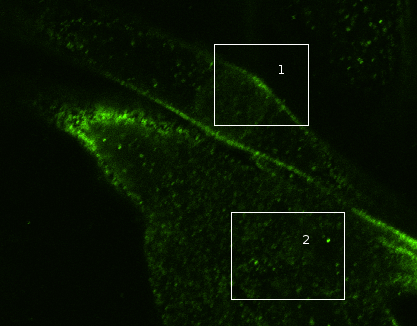
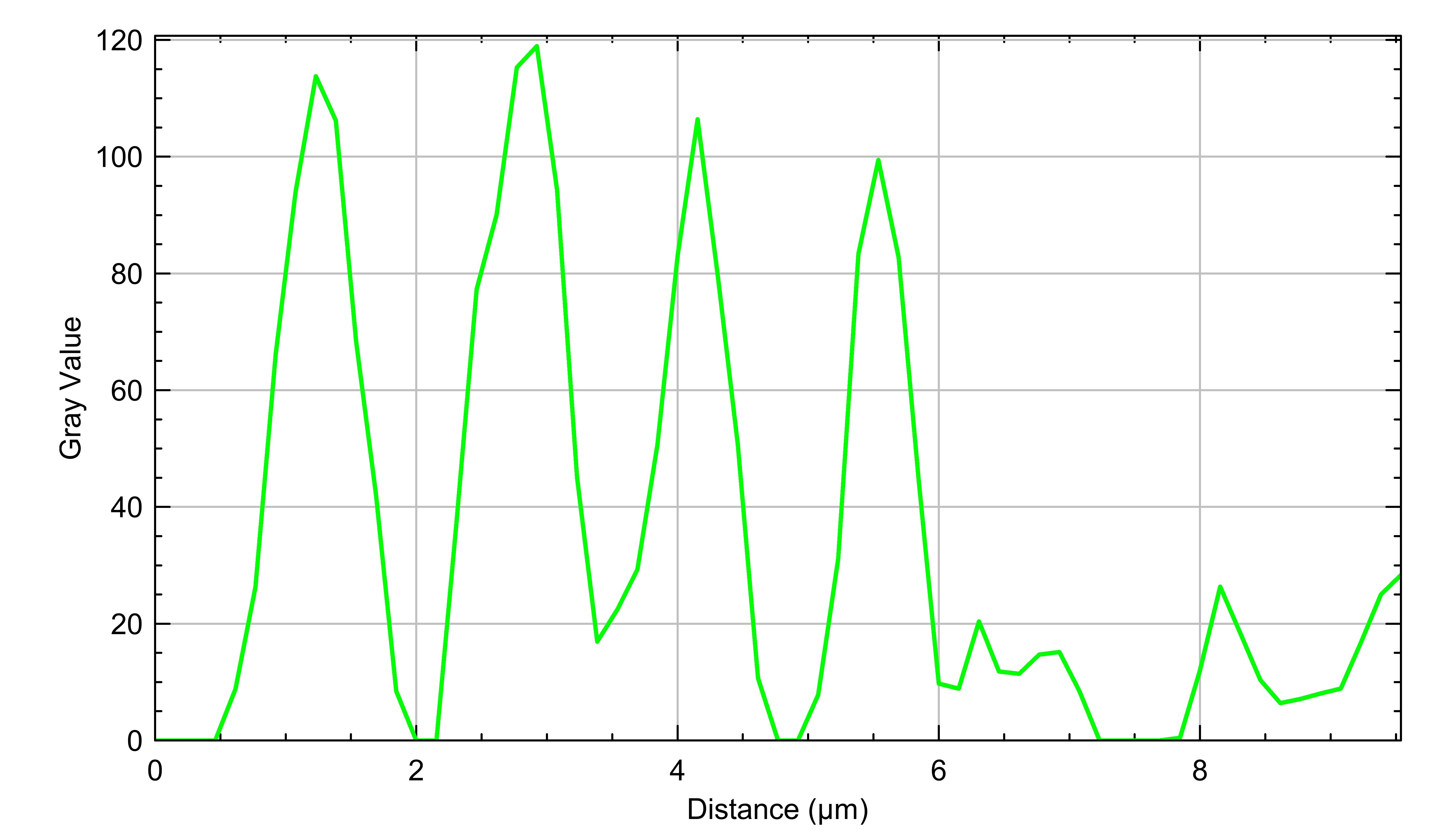
NRVMs were treated with ISO or vehicle control and stained for MYH14 via immunofluorescence. Fluorescence intensity was quantified using ImageJ after signal thresholding. Subcellular MYH14 puncta were enhanced by subtracting Gaussian-blurred images (radius = 1 and 2). Line scans along the periphery identified puncta as peaks with normalized mean gray value ≥ 0.5. Group comparisons were performed using Mann-Whitney U tests.
Results:
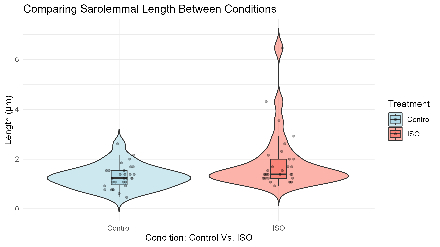
ISO-treated NRVMs exhibited significantly higher MYH14 signal intensity compared to controls (W = 12, p = 0.029). MYH14 localized to both filamentous and punctate structures. Quantitative analysis revealed increased spacing between filament-associated MYH14 punctae in ISO-treated cells (ISO mean = 1856 nm; control = 1287 nm; W = 290, p = 0.0059), consistent with elongation of sarcomeric units.
Conclusion:
ISO treatment induces both increased MYH14 expression and expansion of MYH14 filament spacing, suggesting structural remodeling of sarcomeric units. These findings support a role for MYH14 in mediating cardiac remodeling under hypertrophic stress. Unlike non-cardiac cells, MYH14 puncta in cardiomyocytes maintain periodicity along different axes, reflecting unique myocardial architecture. Ongoing studies aim to define the timeline and upstream signals regulating MYH14 in ISO-induced hypertrophy.
Myh14, encoding non-muscle myosin IIC (NMIIC), is a proposed negative regulator of isoproterenol (ISO)-induced cardiac hypertrophy. ISO upregulated mRNA transcript level of Myh14 in NRVM. In vivo, Myh14 knockout (KO) mice exhibit exaggerated ISO-induced left ventricular (LV) hypertrophy. In neonatal rat ventricular myocytes (NRVMs), Myh14 knockdown leads to poor adhesion and survival, but its protein localization and organization in cardiomyocytes remain unexplored. Based on findings in non-cardiac cells, MYH14 can organize into either filaments or puncta, with punctate separation potentially reflecting sarcomeric length. We aimed to define MYH14 subcellular organization and its changes under hypertrophic stress.
Methods:
NRVMs were treated with ISO or vehicle control and stained for MYH14 via immunofluorescence. Fluorescence intensity was quantified using ImageJ after signal thresholding. Subcellular MYH14 puncta were enhanced by subtracting Gaussian-blurred images (radius = 1 and 2). Line scans along the periphery identified puncta as peaks with normalized mean gray value ≥ 0.5. Group comparisons were performed using Mann-Whitney U tests.
Results:
ISO-treated NRVMs exhibited significantly higher MYH14 signal intensity compared to controls (W = 12, p = 0.029). MYH14 localized to both filamentous and punctate structures. Quantitative analysis revealed increased spacing between filament-associated MYH14 punctae in ISO-treated cells (ISO mean = 1856 nm; control = 1287 nm; W = 290, p = 0.0059), consistent with elongation of sarcomeric units.
Conclusion:
ISO treatment induces both increased MYH14 expression and expansion of MYH14 filament spacing, suggesting structural remodeling of sarcomeric units. These findings support a role for MYH14 in mediating cardiac remodeling under hypertrophic stress. Unlike non-cardiac cells, MYH14 puncta in cardiomyocytes maintain periodicity along different axes, reflecting unique myocardial architecture. Ongoing studies aim to define the timeline and upstream signals regulating MYH14 in ISO-induced hypertrophy.
More abstracts on this topic:
An Overlap Between Takotsubo Cardiomyopathy and Hypertrophic Obstructive Cardiomyopathy Causing Dynamic Left Ventricular Outflow Tract Obstruction: A Unique Case Report
Sharif Muhammad Hammad, Latifi Ahmad Nawid, Naeem Nauman, Baibhav Bipul, Khaleeque Madeeha, Sanjeevi Aditya
9-Year Longitudinal Assessment of the 12-lead Electrocardiogram of Volunteer FirefightersBae Alexander, Dzikowicz Dillon, Lai Chi-ju, Brunner Wendy, Krupa Nicole, Carey Mary, Tam Wai Cheong, Yu Yichen