Final ID: MP14
Genetic and Brain MRI Markers Reveal Cardiovascular Burden Across Brain Disorders
Abstract Body (Do not enter title and authors here): Background:
Cardiovascular disease (CVD) impairs not only cardiac function but also induces structural and functional changes in the brain. However, the specific brain regions most vulnerable to cardiovascular burden and how these map to genetic risk remain poorly characterized. We hypothesized that a machine learning framework trained on brain MRIs across multiple neurologic conditions could identify conserved neurocardiac imaging signatures linked to cardiovascular dysfunction and genetic risk.
Hypothesis:
Integrating brain MRI features with polygenic risk scores (PRS) for CVD will reveal shared brain regions whose structural alterations are predictive of cardiovascular phenotypes and systemic disease burden.
Methods:
We analyzed 1,100 individuals from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) with both structural MRI and genotype data, and incorporated additional MRIs from OASIS (vascular dementia), PPMI (Parkinson’s disease), and BrainWeb (simulated normals). Each image was annotated with a Genetics-Informed Cardiovascular Burden (GIVB) score, integrating PRS for heart failure (HF), atrial fibrillation (AF), and thoracic aortic aneurysm (TAA), along with comorbidity-adjusted blood pressure and heart failure history. A deep convolutional neural network (CNN) was trained to classify GIVB burden; feature importance was extracted using SHAP values and validated with linear modeling.
Results:
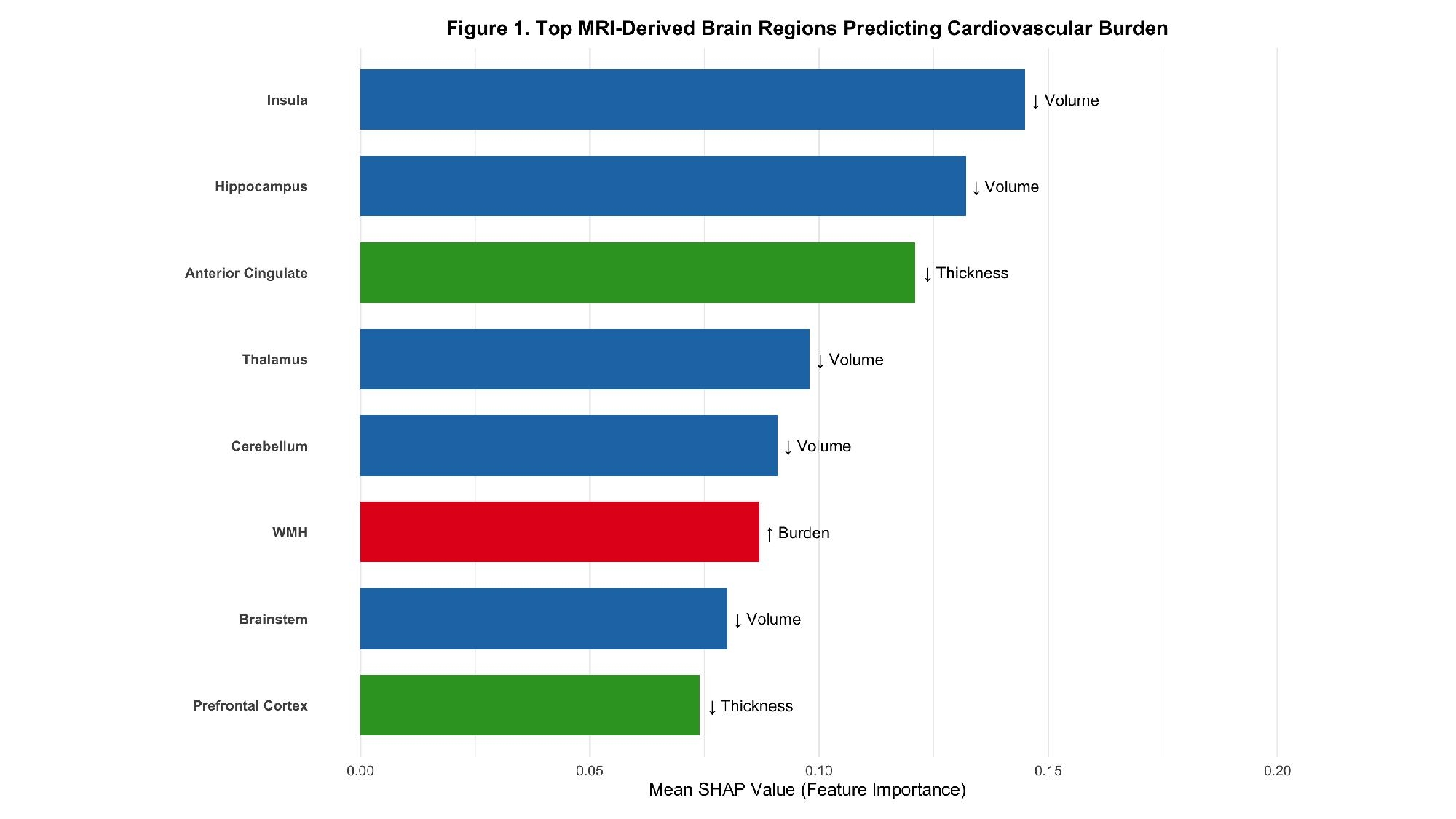
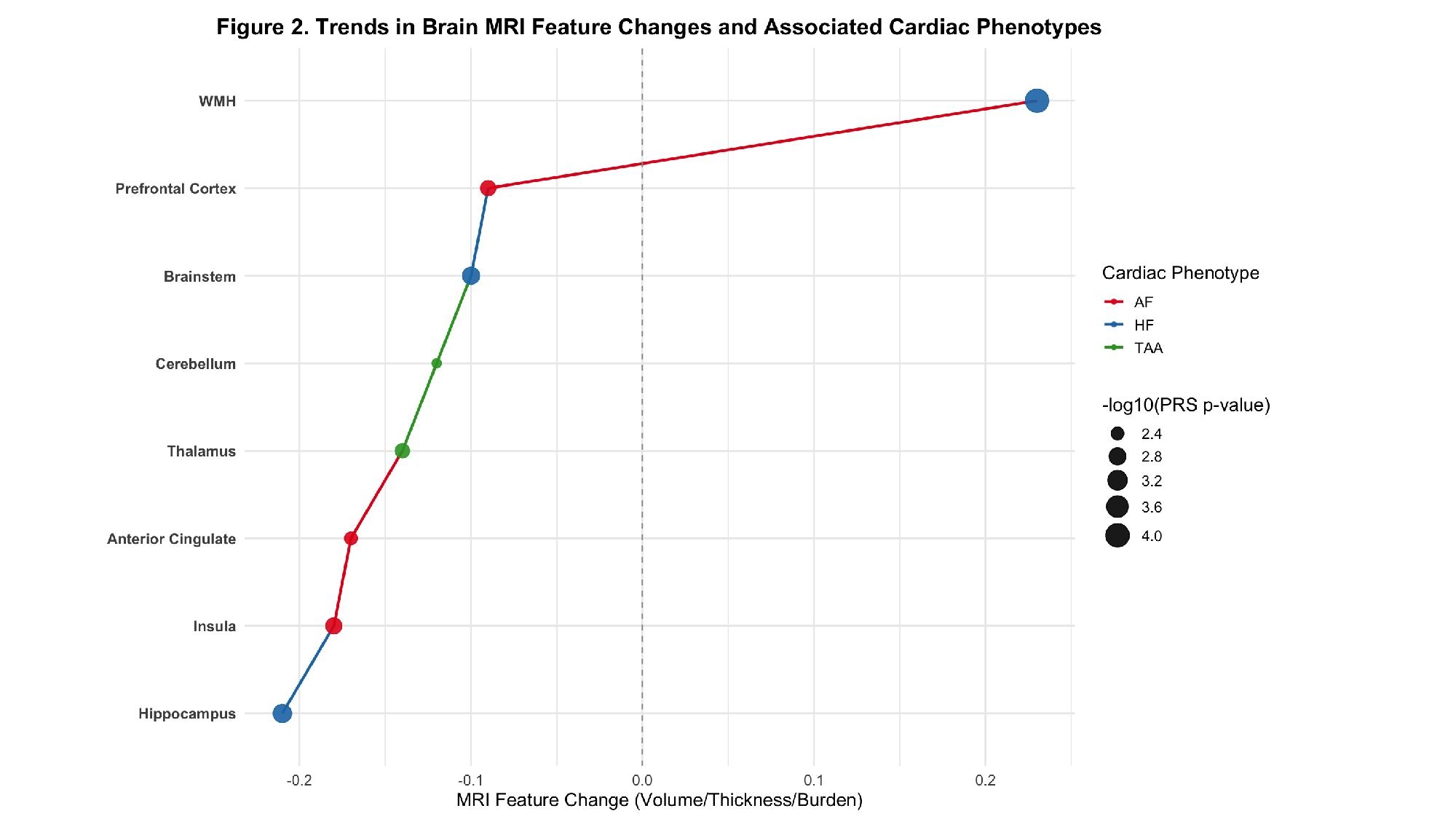
The model achieved 83.6% classification accuracy (AUC = 0.91). As shown in Figure 1, the top predictive regions included the insula, hippocampus, anterior cingulate, thalamus, cerebellum, brainstem, and white matter hyperintensity (WMH) burden. In ADNI, hippocampal volume loss was significantly associated with high HF PRS (β = –0.21, SE = 0.07, p = 0.001), and insular thinning correlated with elevated AF PRS and systolic BP (p < 0.005). WMH burden was highest among HF patients with vascular dementia (p < 0.0001). Figure 2 illustrates directional trends in structural brain changes, each linked to a distinct cardiac phenotype, demonstrating that brain structural changes may serve as early and quantifiable indicators of cardiovascular dysfunction.
Conclusion:
Our findings reveal a neurocardiac imaging-genomic signature linking brain atrophy and white matter burden with cardiovascular disease through shared genetic and autonomic pathways. The brain emerges as a sensitive indicator of systemic heart health, supporting its role in early, multi-organ risk prediction.
Cardiovascular disease (CVD) impairs not only cardiac function but also induces structural and functional changes in the brain. However, the specific brain regions most vulnerable to cardiovascular burden and how these map to genetic risk remain poorly characterized. We hypothesized that a machine learning framework trained on brain MRIs across multiple neurologic conditions could identify conserved neurocardiac imaging signatures linked to cardiovascular dysfunction and genetic risk.
Hypothesis:
Integrating brain MRI features with polygenic risk scores (PRS) for CVD will reveal shared brain regions whose structural alterations are predictive of cardiovascular phenotypes and systemic disease burden.
Methods:
We analyzed 1,100 individuals from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) with both structural MRI and genotype data, and incorporated additional MRIs from OASIS (vascular dementia), PPMI (Parkinson’s disease), and BrainWeb (simulated normals). Each image was annotated with a Genetics-Informed Cardiovascular Burden (GIVB) score, integrating PRS for heart failure (HF), atrial fibrillation (AF), and thoracic aortic aneurysm (TAA), along with comorbidity-adjusted blood pressure and heart failure history. A deep convolutional neural network (CNN) was trained to classify GIVB burden; feature importance was extracted using SHAP values and validated with linear modeling.
Results:
The model achieved 83.6% classification accuracy (AUC = 0.91). As shown in Figure 1, the top predictive regions included the insula, hippocampus, anterior cingulate, thalamus, cerebellum, brainstem, and white matter hyperintensity (WMH) burden. In ADNI, hippocampal volume loss was significantly associated with high HF PRS (β = –0.21, SE = 0.07, p = 0.001), and insular thinning correlated with elevated AF PRS and systolic BP (p < 0.005). WMH burden was highest among HF patients with vascular dementia (p < 0.0001). Figure 2 illustrates directional trends in structural brain changes, each linked to a distinct cardiac phenotype, demonstrating that brain structural changes may serve as early and quantifiable indicators of cardiovascular dysfunction.
Conclusion:
Our findings reveal a neurocardiac imaging-genomic signature linking brain atrophy and white matter burden with cardiovascular disease through shared genetic and autonomic pathways. The brain emerges as a sensitive indicator of systemic heart health, supporting its role in early, multi-organ risk prediction.
More abstracts on this topic:
Accelerated Biological Aging, Early-Life Exposure to Tobacco, and Incident Aortic Aneurysm: A Large-Scale Prospective Cohort Study in UK Biobank
Yang Miaomiao, Feng Weijing, Dang Aimin, Gu Yingzhen
2-Methoxyestradiol By Inhibiting Central Action of 12S-Hydroxyeicosatetraenoic Acid Protects Ovariectomized Mice From HypertensionDutta Shubha, Singh Purnima, Song Chi Young, Shin Ji Soo, Malik Kafait