Final ID: Sa2081
Clinical Outcomes associated with FDA-Labeled and Non-FDA-Labeled Direct Oral Anticoagulant Dosing in US Patients with Atrial Fibrillation, Stratified by Gender: A Real-World Data Study
Abstract Body (Do not enter title and authors here): Background
Atrial fibrillation (AF) is a prevalent condition impacting millions globally. It poses significant thrombotic risks, ranging from stroke to sudden cardiac death, thus understanding current anticoagulant treatment patterns is critical to optimizing outcomes.
Purpose
Study objectives were to gain insight into current anticoagulant treatment landscape and patient characteristics across several AF cohorts, classified by treatment options: no treatment, warfarin, and direct oral anticoagulants (DOACs) as per US prescribing information (standard dose (SD) per label, SD not per label, low dose (LD) per label and LD not per label). Clinical outcomes were compared between patients using DOACs as SD per label and LD not per label overall and by gender.
Methods
This was a retrospective, observational study utilizing Optum’s de-identified Market Clarity Data (Optum® Market Clarity, from January 2017 to September 2024. It only included patients eligible for OAC according to 2019 AHA/ACC/HRS guidelines. A 180-day window was used to assess treatment patterns. Propensity Score Matching including Mahalanobis distance method were used to balance patient characteristics. Cox regression analysis was conducted to calculate hazard ratios for outcomes including stroke, major bleeding (MB), and major adverse cardiac events (MACE, composite of stroke, myocardial infarction, and cardiovascular-related death).
Results
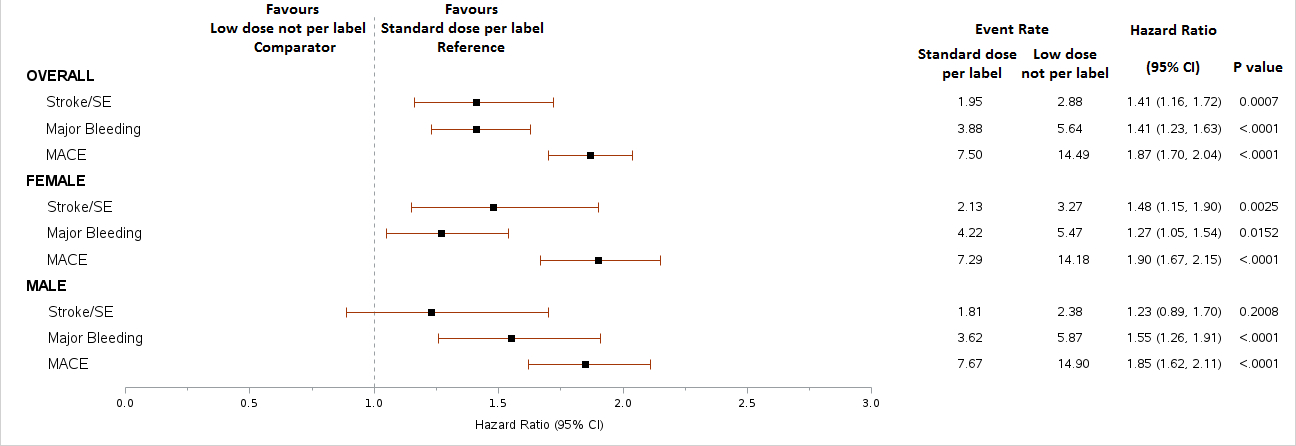
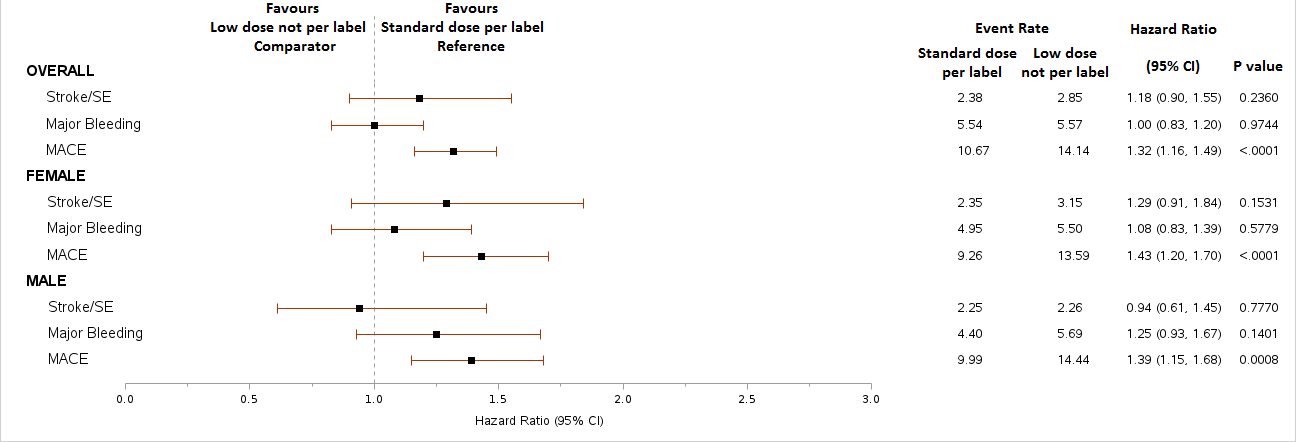
After applying inclusion criteria, the study included 701,536 patients, of whom 37% received DOACs, 11% warfarin, and 52% no OAC therapy. Further exclusions (prior OAC use and invalid bodyweight/serum creatinine) in the DOAC group resulted in a final sample of 48,901 patients. Among these, 78% received SD DOACs per label, 4% SD not per label, 9% LD per label, and 9% LD not per label. These trends were consistent across genders. Overall, patients with LD DOAC not per label were associated with higher rates of MACE, similar rates of stroke/SE and similar rates of MB vs. SD patients. Higher rates of MACE with LD not per label were evident in both males and females.
Conclusion
More than half of AF patients in the US, regardless of gender, who were clinically eligible did not receive anticoagulants. Although some patients were underdosed with DOACs, they did not have a lower risk of bleeding and their MACE event tended to be worse. These findings suggest the need for therapies that reduce thrombosis while minimizing bleeding risk to optimize patient outcomes.
Atrial fibrillation (AF) is a prevalent condition impacting millions globally. It poses significant thrombotic risks, ranging from stroke to sudden cardiac death, thus understanding current anticoagulant treatment patterns is critical to optimizing outcomes.
Purpose
Study objectives were to gain insight into current anticoagulant treatment landscape and patient characteristics across several AF cohorts, classified by treatment options: no treatment, warfarin, and direct oral anticoagulants (DOACs) as per US prescribing information (standard dose (SD) per label, SD not per label, low dose (LD) per label and LD not per label). Clinical outcomes were compared between patients using DOACs as SD per label and LD not per label overall and by gender.
Methods
This was a retrospective, observational study utilizing Optum’s de-identified Market Clarity Data (Optum® Market Clarity, from January 2017 to September 2024. It only included patients eligible for OAC according to 2019 AHA/ACC/HRS guidelines. A 180-day window was used to assess treatment patterns. Propensity Score Matching including Mahalanobis distance method were used to balance patient characteristics. Cox regression analysis was conducted to calculate hazard ratios for outcomes including stroke, major bleeding (MB), and major adverse cardiac events (MACE, composite of stroke, myocardial infarction, and cardiovascular-related death).
Results
After applying inclusion criteria, the study included 701,536 patients, of whom 37% received DOACs, 11% warfarin, and 52% no OAC therapy. Further exclusions (prior OAC use and invalid bodyweight/serum creatinine) in the DOAC group resulted in a final sample of 48,901 patients. Among these, 78% received SD DOACs per label, 4% SD not per label, 9% LD per label, and 9% LD not per label. These trends were consistent across genders. Overall, patients with LD DOAC not per label were associated with higher rates of MACE, similar rates of stroke/SE and similar rates of MB vs. SD patients. Higher rates of MACE with LD not per label were evident in both males and females.
Conclusion
More than half of AF patients in the US, regardless of gender, who were clinically eligible did not receive anticoagulants. Although some patients were underdosed with DOACs, they did not have a lower risk of bleeding and their MACE event tended to be worse. These findings suggest the need for therapies that reduce thrombosis while minimizing bleeding risk to optimize patient outcomes.
More abstracts on this topic:
Anticoagulation versus Antiplatelets in Coronary Artery Ectasia and Acute Coronary Syndrome: A Systematic Review and Meta-analysis
Hernandez-pastrana Sarai, Latapi Ruiz Esparza Ximena, Martignoni Felipe, Araiza Diego, Doma Mohamed, Fatima Syeda Rubab, Hemdanieh Maya, Kritya Mangesh, Huang Wilbert, Naji Zahra, Lingamsetty Shanmukh Sai Pavan, Gewehr Douglas
A-band titin-truncating variant promotes the development of arrhythmia-induced cardiomyopathy in a novel genetically-engineered porcine modelLee Kwonjae, Del Rio Carlos, Mcnally Elizabeth, Pfenniger Anna, Bhatnagar Ashita, Glinton Kristofor, Burrell Amy, Ober Rebecca, Mcluckie Alicia, Bishop Brian, Rogers Christopher, Geist Gail