Final ID: MP2397
Compliance, ECG quality, and engagement with a smartphone app in patients with in-clinic compared with home-based, self-applied long-term continuous ECG patch monitors
Abstract Body (Do not enter title and authors here): Background
Long-term continuous ambulatory cardiac monitoring (LTCM) is a widely used diagnostic tool for arrhythmia detection, outperforming other modalities. COVID-19 accelerated adoption of home enrollment (HE) for LTCM, which includes mailing devices to patients for self-application and activation, highlighting the need for patient-centered solutions that optimize usability and comfort. HE was recently made available for a next-generation LTCM, which is smaller and lighter than prior designs, with a breathable adhesive, and has demonstrated superior performance.
Aims
We assessed wear compliance and ECG signal quality for next generation LTCM devices applied in-clinic by a technician vs. HE. Additionally, we evaluated the impact of a smartphone app on wear compliance and ECG quality.
Methods
U.S. adults prescribed the Zio Monitor (iRhythm Technologies, San Francisco, CA) for 14 days between December 2, 2024 - March 16, 2025, were included, corresponding to the initial availability of HE for Zio Monitor. Outcomes compared between in-clinic and HE devices included mean wear time, mean analyzable time (% free from artifact), early wear terminations (≤ 2 days), and actionable arrhythmia yield. Additional analyses evaluated outcomes among patients opting to use a smartphone app (MyZio), which provides onboarding, digitized instructions, and reminders for wear and return, vs. those who did not.
Results
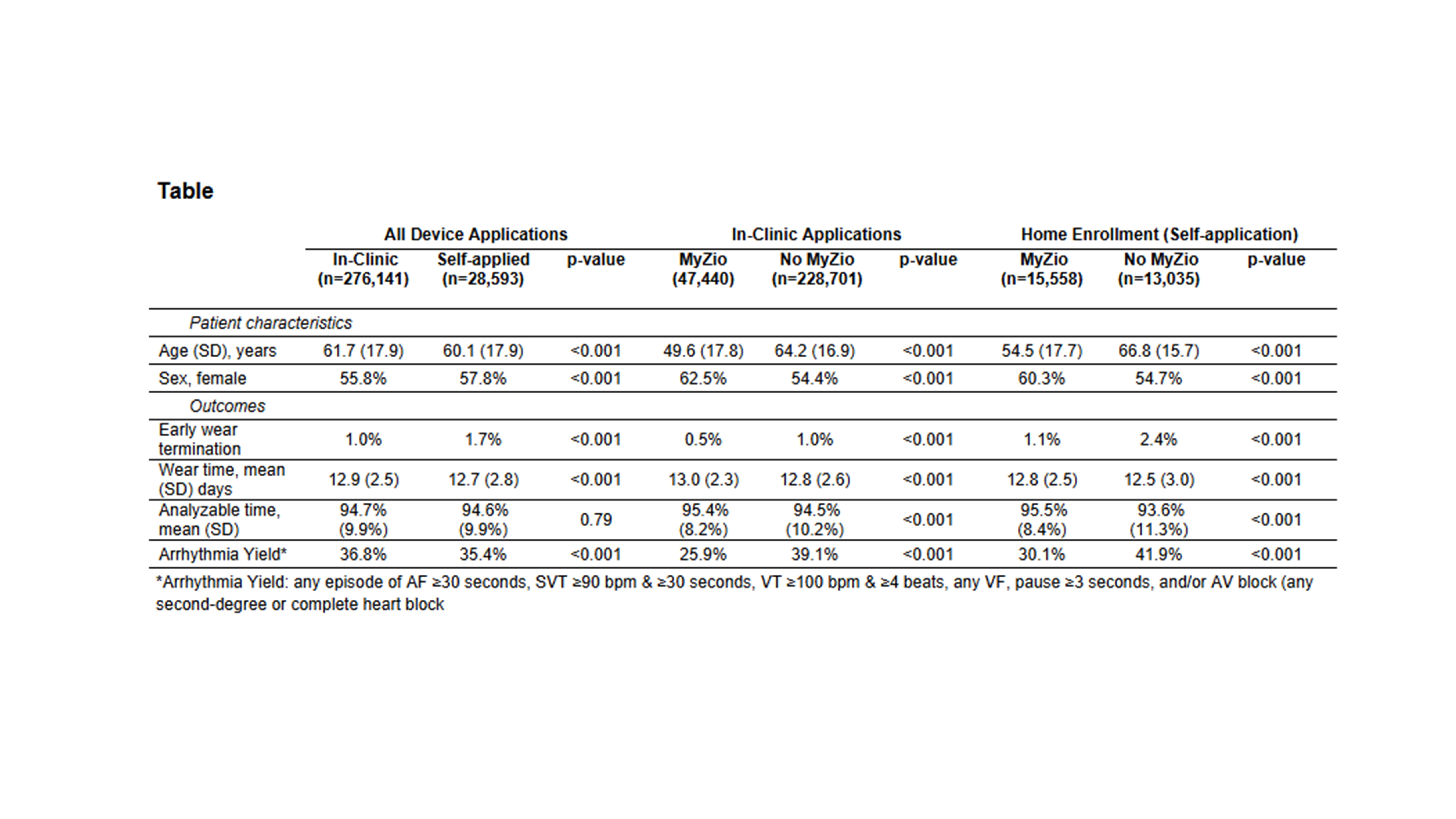
Of 304,735 LTCM devices worn, 276,142 (91.6%) were applied in-clinic and 28,593 (9.4%) were HE. Mean age was 61.5±17.9 years; 56.0% were female. App use was higher in the HE group (54% vs 17%, p < 0.0001). Mean wear time and % analyzable time were high and comparable for in-clinic and HE. Early wear terminations were infrequent in both groups and arrhythmia yield was comparable. App use was associated with lower % of early wear terminations and greater analyzable time in both groups (Table). Among prescribed devices, return compliance (activated, worn and returned ≤ 45 days) was higher in app users for both in-clinic (96.0% vs. 93.2%) and HE (90.4% vs. 71.1%) devices.
Conclusion
Wear compliance and percent analyzable time for a next-generation LTCM were high and comparable when applied in-clinic by a technician vs. HE, indicating that HE achieves comparable arrhythmia detection while eliminating in-clinic visits and reducing provider burden. Patient apps as medical device adjuncts may further improve enrollment and compliance with home-based or ambulatory diagnostics.
Long-term continuous ambulatory cardiac monitoring (LTCM) is a widely used diagnostic tool for arrhythmia detection, outperforming other modalities. COVID-19 accelerated adoption of home enrollment (HE) for LTCM, which includes mailing devices to patients for self-application and activation, highlighting the need for patient-centered solutions that optimize usability and comfort. HE was recently made available for a next-generation LTCM, which is smaller and lighter than prior designs, with a breathable adhesive, and has demonstrated superior performance.
Aims
We assessed wear compliance and ECG signal quality for next generation LTCM devices applied in-clinic by a technician vs. HE. Additionally, we evaluated the impact of a smartphone app on wear compliance and ECG quality.
Methods
U.S. adults prescribed the Zio Monitor (iRhythm Technologies, San Francisco, CA) for 14 days between December 2, 2024 - March 16, 2025, were included, corresponding to the initial availability of HE for Zio Monitor. Outcomes compared between in-clinic and HE devices included mean wear time, mean analyzable time (% free from artifact), early wear terminations (≤ 2 days), and actionable arrhythmia yield. Additional analyses evaluated outcomes among patients opting to use a smartphone app (MyZio), which provides onboarding, digitized instructions, and reminders for wear and return, vs. those who did not.
Results
Of 304,735 LTCM devices worn, 276,142 (91.6%) were applied in-clinic and 28,593 (9.4%) were HE. Mean age was 61.5±17.9 years; 56.0% were female. App use was higher in the HE group (54% vs 17%, p < 0.0001). Mean wear time and % analyzable time were high and comparable for in-clinic and HE. Early wear terminations were infrequent in both groups and arrhythmia yield was comparable. App use was associated with lower % of early wear terminations and greater analyzable time in both groups (Table). Among prescribed devices, return compliance (activated, worn and returned ≤ 45 days) was higher in app users for both in-clinic (96.0% vs. 93.2%) and HE (90.4% vs. 71.1%) devices.
Conclusion
Wear compliance and percent analyzable time for a next-generation LTCM were high and comparable when applied in-clinic by a technician vs. HE, indicating that HE achieves comparable arrhythmia detection while eliminating in-clinic visits and reducing provider burden. Patient apps as medical device adjuncts may further improve enrollment and compliance with home-based or ambulatory diagnostics.
More abstracts on this topic:
Addressing Racial Bias in GPT-4 Cardiovascular Clinical Reasoning
Krieger Katherine, Rossi Camilla, Rahouma Mohamed, Gaudino Mario, Hameed Irbaz, Quer Giorgio, Mack Charles, Savic Marco, Mantaj Polina, Hirofuji Aina, Gregg Alexander, Soletti Giovanni
Acute Hemodynamic Effects of Sotatercept in Pulmonary Arterial HypertensionKremer Nils, Naeije Robert, Ghofrani Ardeschir, Tello Khodr, Thal Bruno, Janetzko Patrick, Yogeswaran Athiththan, Rako Zvonimir, Pullamsetti Soni, Bonnet Sebastien, Seeger Werner, Grimminger Friedrich