Final ID: Sa2097
Feasibility of point-of-wear patient satisfaction surveys to validate patient-centered product enhancements: results from over 300,000 patients for long-term ambulatory cardiac monitoring
Abstract Body (Do not enter title and authors here): Introduction
Although 14-day patch-based long-term continuous ambulatory ECG monitoring (LTCM) has shown greater diagnostic yield and lower retest rates compared to other rhythm monitoring modalities, wear can still be limited by factors related to patient comfort and acceptance. Rather than data from small, non-generalizable focus groups, patient survey data at point of care offered to all patients may be valuable in collecting quality improvement data on product experience and satisfaction. We assessed the feasibility of this approach to compare patient satisfaction associated with the prior generation LTCM to that of a new generation, FDA-cleared LTCM product designed with patient-centered features, including a more breathable adhesive, waterproof housing, thinner profile, and lighter weight.
Methods
Starting in March 2018, we implemented a survey provided to all patients prescribed Zio® XT LTCM (iRhythm Technologies, San Francisco, CA) to complete and return at end of wear. The survey was completed via paper card or digitally via a web address printed on the card. The survey included questions regarding ease of use, comfort, ability for normal activity, and willingness to wear the device again. Scores of 4 or 5 (i.e., Agree or Strongly Agree) on a Likert scale were considered affirmative responses. Beginning in April 2022, the new Zio® Monitor device was launched for use and the same survey method was used. We compared survey responses for Monitor and XT between Jan 1 and Dec 31, 2023.
Results
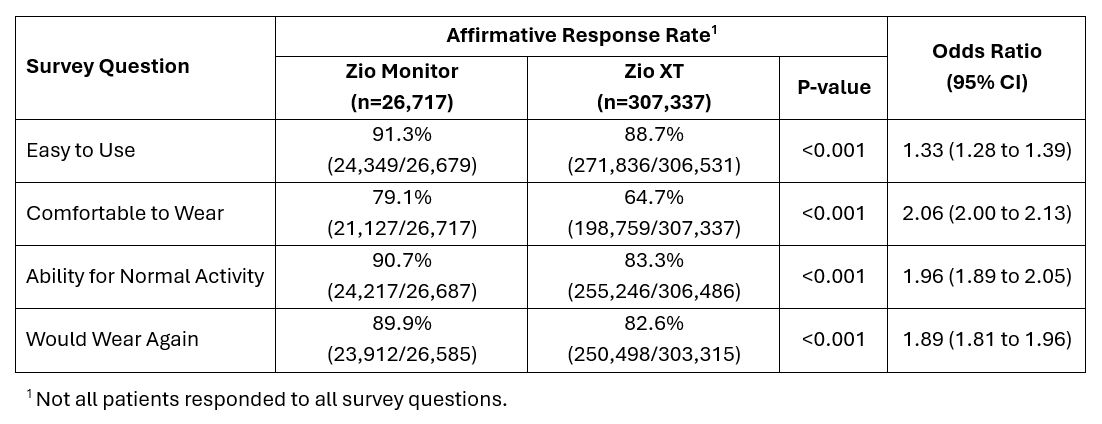
Among 334,054 respondents, the new LTCM was associated with a greater proportion of affirmative responses across all survey categories (Table 1), with the largest gains in comfort of wear (79.1% vs. 64.7%; p<0.001), ability for normal activity (90.7% vs. 83.3%; p<0.001) and willingness to wear again (89.9% vs. 82.6%; p<0.001).
Conclusion
Patient survey data for post-market quality assessment is feasible for digital health technologies, in this case leading to over 300,000 total respondents in one year. Measures of patient satisfaction were higher with the new device, which may be due to patient-centered product enhancements.z
Although 14-day patch-based long-term continuous ambulatory ECG monitoring (LTCM) has shown greater diagnostic yield and lower retest rates compared to other rhythm monitoring modalities, wear can still be limited by factors related to patient comfort and acceptance. Rather than data from small, non-generalizable focus groups, patient survey data at point of care offered to all patients may be valuable in collecting quality improvement data on product experience and satisfaction. We assessed the feasibility of this approach to compare patient satisfaction associated with the prior generation LTCM to that of a new generation, FDA-cleared LTCM product designed with patient-centered features, including a more breathable adhesive, waterproof housing, thinner profile, and lighter weight.
Methods
Starting in March 2018, we implemented a survey provided to all patients prescribed Zio® XT LTCM (iRhythm Technologies, San Francisco, CA) to complete and return at end of wear. The survey was completed via paper card or digitally via a web address printed on the card. The survey included questions regarding ease of use, comfort, ability for normal activity, and willingness to wear the device again. Scores of 4 or 5 (i.e., Agree or Strongly Agree) on a Likert scale were considered affirmative responses. Beginning in April 2022, the new Zio® Monitor device was launched for use and the same survey method was used. We compared survey responses for Monitor and XT between Jan 1 and Dec 31, 2023.
Results
Among 334,054 respondents, the new LTCM was associated with a greater proportion of affirmative responses across all survey categories (Table 1), with the largest gains in comfort of wear (79.1% vs. 64.7%; p<0.001), ability for normal activity (90.7% vs. 83.3%; p<0.001) and willingness to wear again (89.9% vs. 82.6%; p<0.001).
Conclusion
Patient survey data for post-market quality assessment is feasible for digital health technologies, in this case leading to over 300,000 total respondents in one year. Measures of patient satisfaction were higher with the new device, which may be due to patient-centered product enhancements.z
More abstracts on this topic:
Despite lower prevalence of hypertension, women have less blood pressure control than men: findings from a large electronic health-based cohort
Rastogi Aman, Behuria Supreeti, Chen Melanie, Abdallah Ramsey, Parikh Nisha
Impact of Myocardial Injury Assessment in Emergency Department on Disposition Distribution and Time to DispositionLandry Alexander, Defilippis Andrew, Michael Kirolos, Lidani Karita, Palmer Benjamin, Tomar Shubham, Xu Meng, Libre Michael, Sexton Mitchell, Wrenn Jesse