Final ID: MP756
Donor Renal Function Should Inform Evaluation of Circulatory Death Heart Offers
Abstract Body (Do not enter title and authors here): Background:
Donation after circulatory death (DCD) is the fastest-growing source of donor hearts in the United States, yet the impact of donor hypertension, diabetes mellitus, and severe renal dysfunction on 1-year recipient survival remains unclear. While earlier analyses in brain-dead donors suggested that hypertension, diabetes, and moderate renal impairment did not worsen short-term outcomes, these findings have not been systematically revisited in the physiologically distinct setting of DCD heart transplantation
Methods:
Adult (≥18 y) recipients of isolated DCD heart transplants in the UNOS registry (January 2019- April 2025; n = 2,058) were studied. Donors were stratified by HTN, DM, and estimated glomerular filtration rate (eGFR); severe dysfunction was prespecified as eGFR < 45 One-year all-cause mortality was the primary endpoint. Associations were tested with Kaplan–Meier curves, multivariable Cox regression, and 1:1 propensity-score matching (caliper = 0.20 SD, 198 pairs). A parallel donation-after-brain-death (DBD) cohort provided context.
Results:
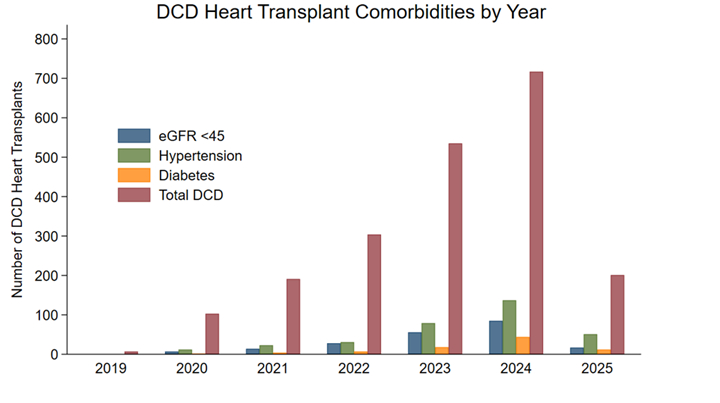
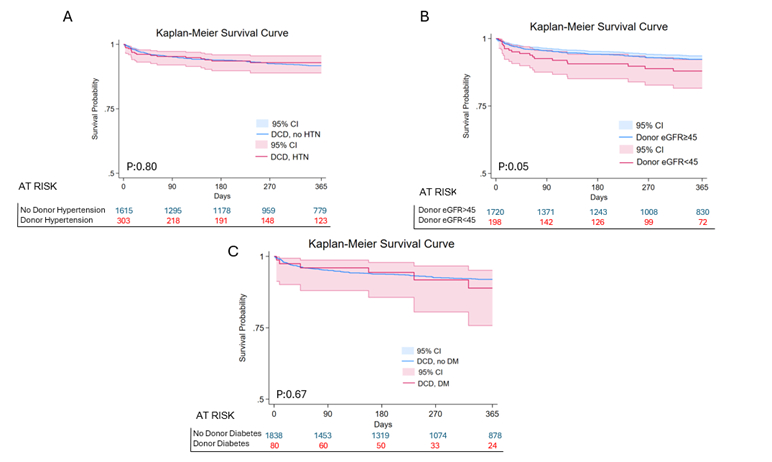
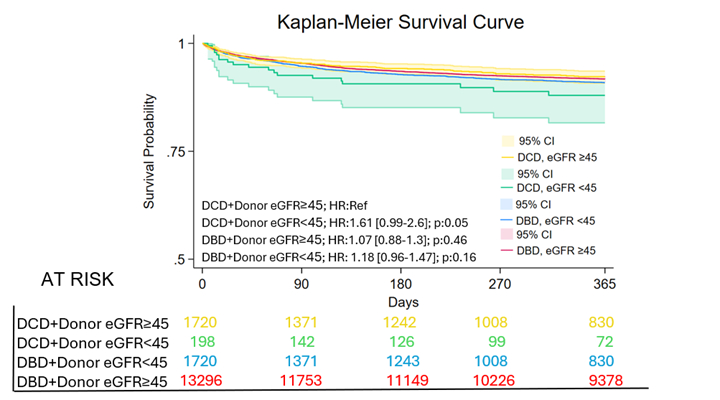
DCD activity increased steadily, accompanied by growing acceptance of donors with HTN, DM, and low eGFR (Image 1). One-year survival was unchanged by HTN (88.1 % vs 88.3 %, p = 0.80) or DM (87.4 % vs 88.2 %, p = 0.67) (Image 2). In contrast, recipients of grafts from donors with eGFR < 45 experienced lower survival (82.6 % vs 88.6 %, p = 0.04); this difference remained significant after 1:1 propensity matching (p = 0.009). Severe renal dysfunction was independently prognostic in multivariable analysis (HR 1.81, 95 % CI 1.07–3.06, p = 0.028). Across four strata defined by donor type and eGFR, excess mortality emerged only for DCD hearts with low eGFR (adjusted HR 1.87, p = 0.05), whereas renal status did not influence DBD outcomes (Image 3).
Conclusions:
Donor HTN and DM do not compromise 1-year survival in DCD heart transplantation, supporting liberal use of such grafts. Severe donor renal dysfunction, however, nearly doubles early mortality and appears uniquely detrimental in the DCD context, potentially reflecting synergy between inflammatory priming and warm ischemia. Routine incorporation of an eGFR threshold into DCD evaluation algorithms—augmented by contemporary perfusion or regional reperfusion strategies—may enable continued expansion of the donor pool while safeguarding recipient outcomes.
Donation after circulatory death (DCD) is the fastest-growing source of donor hearts in the United States, yet the impact of donor hypertension, diabetes mellitus, and severe renal dysfunction on 1-year recipient survival remains unclear. While earlier analyses in brain-dead donors suggested that hypertension, diabetes, and moderate renal impairment did not worsen short-term outcomes, these findings have not been systematically revisited in the physiologically distinct setting of DCD heart transplantation
Methods:
Adult (≥18 y) recipients of isolated DCD heart transplants in the UNOS registry (January 2019- April 2025; n = 2,058) were studied. Donors were stratified by HTN, DM, and estimated glomerular filtration rate (eGFR); severe dysfunction was prespecified as eGFR < 45 One-year all-cause mortality was the primary endpoint. Associations were tested with Kaplan–Meier curves, multivariable Cox regression, and 1:1 propensity-score matching (caliper = 0.20 SD, 198 pairs). A parallel donation-after-brain-death (DBD) cohort provided context.
Results:
DCD activity increased steadily, accompanied by growing acceptance of donors with HTN, DM, and low eGFR (Image 1). One-year survival was unchanged by HTN (88.1 % vs 88.3 %, p = 0.80) or DM (87.4 % vs 88.2 %, p = 0.67) (Image 2). In contrast, recipients of grafts from donors with eGFR < 45 experienced lower survival (82.6 % vs 88.6 %, p = 0.04); this difference remained significant after 1:1 propensity matching (p = 0.009). Severe renal dysfunction was independently prognostic in multivariable analysis (HR 1.81, 95 % CI 1.07–3.06, p = 0.028). Across four strata defined by donor type and eGFR, excess mortality emerged only for DCD hearts with low eGFR (adjusted HR 1.87, p = 0.05), whereas renal status did not influence DBD outcomes (Image 3).
Conclusions:
Donor HTN and DM do not compromise 1-year survival in DCD heart transplantation, supporting liberal use of such grafts. Severe donor renal dysfunction, however, nearly doubles early mortality and appears uniquely detrimental in the DCD context, potentially reflecting synergy between inflammatory priming and warm ischemia. Routine incorporation of an eGFR threshold into DCD evaluation algorithms—augmented by contemporary perfusion or regional reperfusion strategies—may enable continued expansion of the donor pool while safeguarding recipient outcomes.
More abstracts on this topic:
Antithrombotic Strategies and Outcomes in Neonates and Infants with Cardiac Shunts: A Systematic Review and Meta-analysis
Kiskaddon Amy, Do Nhue, Goldenberg Neil, Betensky Marisol, Branstetter Joshua, Ashour Dina, Williams Pamela, Stock Arabela, Silvey Michael, Giglia Therese
Assessing Racial Disparities in Heart Transplant Allocations Post-2018 Policy ChangeMalkani Kabir, Zhang Ruina, Li Han, Ezema Ashley, Steitieh Diala, Purkayastha Subhanik, Kini Vinay