Final ID: Mo4048
The MSX1-E135D Mutation Disrupts SUMOylation, Interactions with T-box Proteins, and Smooth Muscle Actin Expression
Abstract Body (Do not enter title and authors here): Introduction
We previously reported a putative mutation in Muscle Segment Homeobox 1 (MSX1; p.E135D) in a multigenerational family with arrhythmogenic cardiomyopathy, myocardial fibrosis, valve disease, and sudden death. MSX1 is a transcriptional repressor that interacts with TBX3 and affects the second heart field, cardiac neural crest, and mesenchymal differentiation. Cardiac specific deletion of MSX1 in 4-week-old and 12-week-old mice caused high degree AV block, junctional tachyarrhythmia, and sinus exit block.
Questions
To study potential mechanisms by which MSX1-E135D could alter cardiac structure and function, we assessed its impact on 1) SUMOylation at a consensus SUMOylation site, MSX1-K133, 2) complex formation of MSX1 with T-box transcription factors, and 3) cardiac connective tissue.
Methods
For SUMOylation, MSX1-WT, MSX1-E135D, and MSX1-K15A-K133A were co-transfected with SUMO1 and PIAS1 in HEK293 cells. For T-box interactions, T-box transcription factors were co-transfected in HEK293 cells with FLAG-MSX1-WT or FLAG-MSX1-E135D and immunoprecipitated with anti-FLAG. For connective tissue, neonatal rat cardiac fibroblasts (NRCFs) were incubated with adenoviruses expressing MSX1-WT or E135D followed by TGF-beta (TGF-β) exposure and measurement of alpha smooth muscle actin (α-SMA).
Results
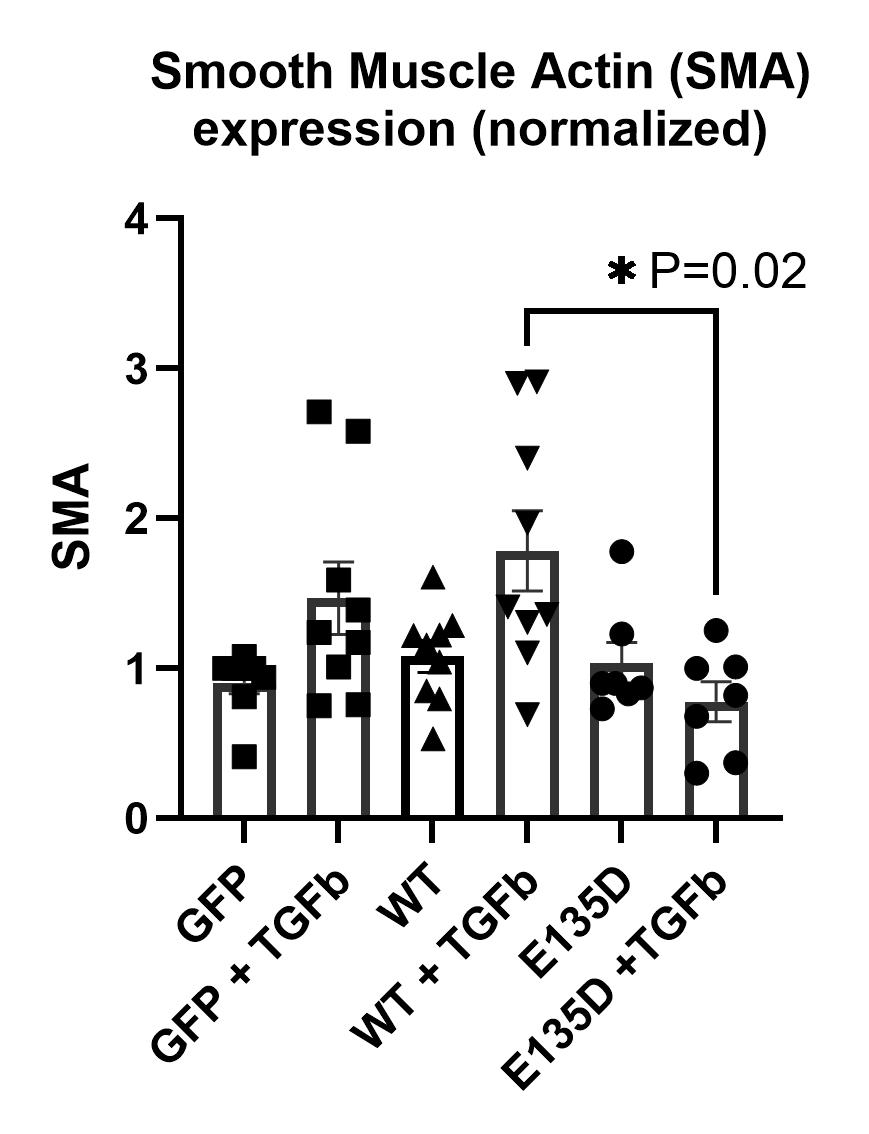
MSX1-WT but not MSX1-E135D could be SUMOylated on Western blot. MSX1-E135D co-immunoprecipitated TBX5 and TBX3 to a lesser degree than WT, while its interaction with TBX2 was unchanged. MSX1-E135D overexpression in neonatal rat cardiac fibroblasts (NRCFs) blocked TGF-β-induced upregulation of alpha smooth muscle actin (see Figure).
Conclusions
At the molecular level, MSX1-E135D appears to impair SUMOylation of MSX1 and weaken interactions with the T-box transcription factors, TBX3 and TBX5. At the developmental level, the mutation disrupts TGF-β pathway signaling, which could perturb the Endothelial to Mesenchymal Transition that drives developmental changes. These findings could underlie the myocardial fibrosis, arrhythmias, and valve disease seen in the family carrying the MSX1-E135D mutation.
We previously reported a putative mutation in Muscle Segment Homeobox 1 (MSX1; p.E135D) in a multigenerational family with arrhythmogenic cardiomyopathy, myocardial fibrosis, valve disease, and sudden death. MSX1 is a transcriptional repressor that interacts with TBX3 and affects the second heart field, cardiac neural crest, and mesenchymal differentiation. Cardiac specific deletion of MSX1 in 4-week-old and 12-week-old mice caused high degree AV block, junctional tachyarrhythmia, and sinus exit block.
Questions
To study potential mechanisms by which MSX1-E135D could alter cardiac structure and function, we assessed its impact on 1) SUMOylation at a consensus SUMOylation site, MSX1-K133, 2) complex formation of MSX1 with T-box transcription factors, and 3) cardiac connective tissue.
Methods
For SUMOylation, MSX1-WT, MSX1-E135D, and MSX1-K15A-K133A were co-transfected with SUMO1 and PIAS1 in HEK293 cells. For T-box interactions, T-box transcription factors were co-transfected in HEK293 cells with FLAG-MSX1-WT or FLAG-MSX1-E135D and immunoprecipitated with anti-FLAG. For connective tissue, neonatal rat cardiac fibroblasts (NRCFs) were incubated with adenoviruses expressing MSX1-WT or E135D followed by TGF-beta (TGF-β) exposure and measurement of alpha smooth muscle actin (α-SMA).
Results
MSX1-WT but not MSX1-E135D could be SUMOylated on Western blot. MSX1-E135D co-immunoprecipitated TBX5 and TBX3 to a lesser degree than WT, while its interaction with TBX2 was unchanged. MSX1-E135D overexpression in neonatal rat cardiac fibroblasts (NRCFs) blocked TGF-β-induced upregulation of alpha smooth muscle actin (see Figure).
Conclusions
At the molecular level, MSX1-E135D appears to impair SUMOylation of MSX1 and weaken interactions with the T-box transcription factors, TBX3 and TBX5. At the developmental level, the mutation disrupts TGF-β pathway signaling, which could perturb the Endothelial to Mesenchymal Transition that drives developmental changes. These findings could underlie the myocardial fibrosis, arrhythmias, and valve disease seen in the family carrying the MSX1-E135D mutation.
More abstracts on this topic:
Can the reduction of mutant endothelial cells hold promise for treating brain arteriovenous malformation?
Shabani Nabikandi Zahra, Prado Leandro, Shaligram Sonali, Zhang Rui, Zhu Wan, Liang Rich, Yadav Alka, Wang Calvin, Su Hua
Impact of Mitral Annular Calcification on Cardiovascular Outcomes in Patients with Diastolic Heart FailureIsaacs Evan, Idowu Abiodun, Pressman Gregg