Final ID: Sa3047
Neonatal hyperoxia disrupts left ventricular vascular development and promotes maladaptive remodeling linked to adult cardiovascular dysfunction.
Abstract Body (Do not enter title and authors here): Introduction:
Prematurity (PT≤37 weeks’ gestation), which affects over 10% of births globally, is associated with systemic alterations in vascular structure and function and increased cardiovascular disease risk. We showed that neonatal hyperoxia, mimicking PT-related conditions, induces long-term cardiac dysfunction in rats (Oxygen-Induced Cardiomyopathy, OIC). However, whether neonatal hyperoxia disrupts the development of the LV left ventricular (LV) vascular network is unknown. We aimed to characterize coronary and capillary morphology and molecular signature in OIC model.
Research Questions :
We hypothesized that neonatal exposure to hyperoxia disrupts LV vascular network development.
Methods:
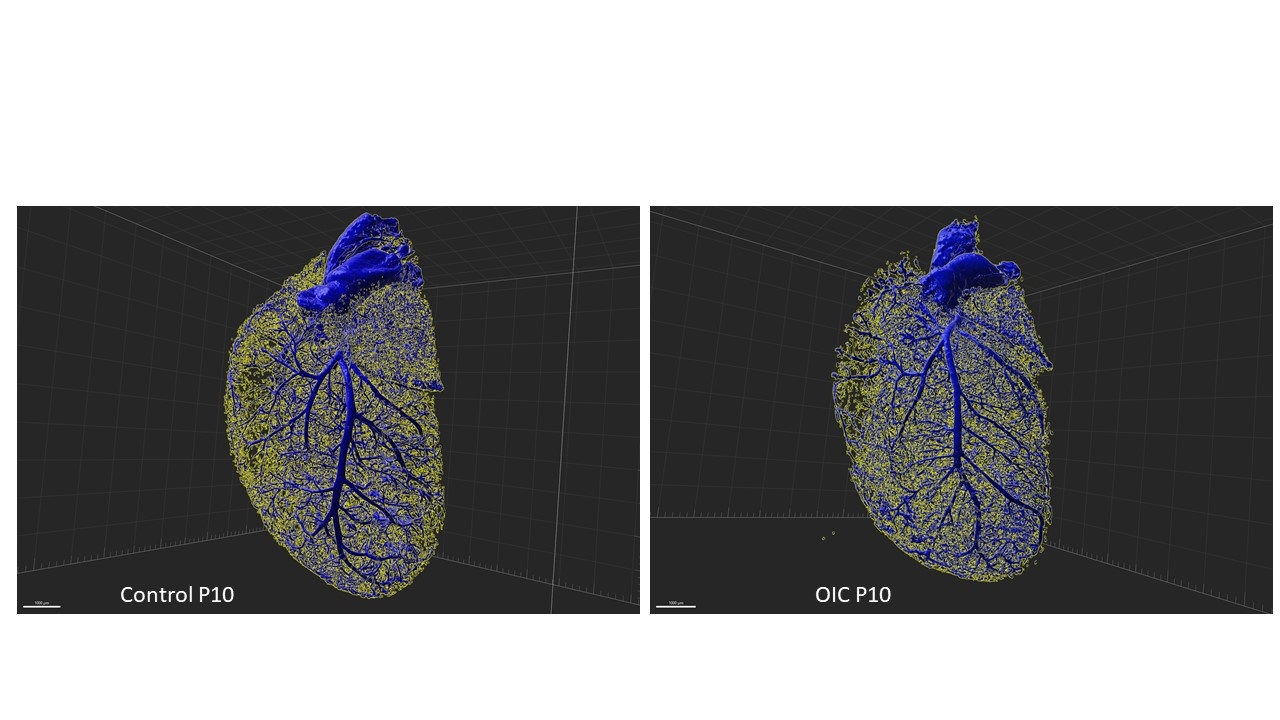
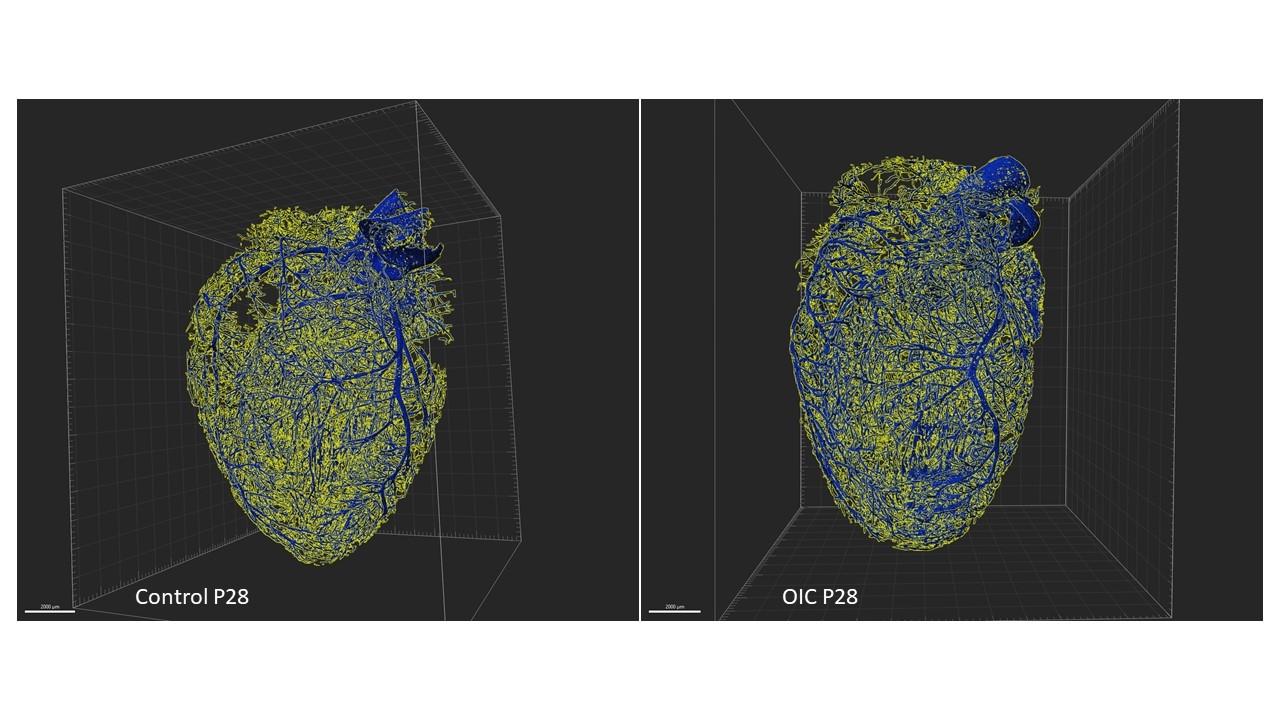
Male pups were kept with their mothers in 80% O (OIC) or room air (CTRL) from postnatal day 3 (P3) to P10. Hearts were collected at P10, 4 and 16 weeks. Capillary density, cardiomyocytes size was measured by immunofluorescence (CD31/WGA). High-resolution 3D reconstruction vascular coronary architecture was achieved by combining fluorescent labelling of coronaries (α-SMA) with a tissue-cleaning protocol (iDisco+) and lightsheet microscope, evaluated with Imaris software. Expression of VEGFA, VEGFB, VEGFR1, VEGFR2, and NRP1 was assessed by RT-qPCR and Western blot. Results (mean±SEM; Ctrl vs OIC) are compared using t test (N = 6/group).
Results:
At P10, OIC exhibited decreased capillary/cardiomyocyte density ratio (0.633±0.020 vs. 0.713±0.020mm2), shorter (77.9±0.12 vs. 83.3±0.08μm) and narrow (45.4±0.04 vs. 49.2±0.03μm) coronary segments, despite increased VEGFA (76%) and NRP1 (38%). At 4 weeks, OIC showed increased arteriolar length (101.2 ± 0.18 vs. 95.5 ± 0.20 μm) and diameter (51.2 ± 0.11 vs. 49.7 ± 0.07 μm), decreased VEGFA (14%) and unchanged capillary density. At 16 weeks, OIC presented increased in cardiomyocytes size (497.0±16.3 vs. 437.9±12.9µm2), and in capillary/cardiomyocyte density ratio (1.599±0.020 vs. 1.466±0.029 mm2) and downregulation of VEGFA (23%), VEGFR1 (27%), VEGFR2 (20%), and NRP1 (17%).
Conclusion:
Neonatal hyperoxia disrupts early LV coronary vascular development and induces long-term maladaptive remodeling. Early capillary rarefaction despite elevated VEGFA along with long-term cardiomyocytes hypertrophy and receptor downregulation suggests disrupt angiogenesis. These results reinforce the distinct alterations elicited by PT-related conditions and enhance the mechanistic understanding of increased susceptibility to cardiac diseases after PT.
Prematurity (PT≤37 weeks’ gestation), which affects over 10% of births globally, is associated with systemic alterations in vascular structure and function and increased cardiovascular disease risk. We showed that neonatal hyperoxia, mimicking PT-related conditions, induces long-term cardiac dysfunction in rats (Oxygen-Induced Cardiomyopathy, OIC). However, whether neonatal hyperoxia disrupts the development of the LV left ventricular (LV) vascular network is unknown. We aimed to characterize coronary and capillary morphology and molecular signature in OIC model.
Research Questions :
We hypothesized that neonatal exposure to hyperoxia disrupts LV vascular network development.
Methods:
Male pups were kept with their mothers in 80% O (OIC) or room air (CTRL) from postnatal day 3 (P3) to P10. Hearts were collected at P10, 4 and 16 weeks. Capillary density, cardiomyocytes size was measured by immunofluorescence (CD31/WGA). High-resolution 3D reconstruction vascular coronary architecture was achieved by combining fluorescent labelling of coronaries (α-SMA) with a tissue-cleaning protocol (iDisco+) and lightsheet microscope, evaluated with Imaris software. Expression of VEGFA, VEGFB, VEGFR1, VEGFR2, and NRP1 was assessed by RT-qPCR and Western blot. Results (mean±SEM; Ctrl vs OIC) are compared using t test (N = 6/group).
Results:
At P10, OIC exhibited decreased capillary/cardiomyocyte density ratio (0.633±0.020 vs. 0.713±0.020mm2), shorter (77.9±0.12 vs. 83.3±0.08μm) and narrow (45.4±0.04 vs. 49.2±0.03μm) coronary segments, despite increased VEGFA (76%) and NRP1 (38%). At 4 weeks, OIC showed increased arteriolar length (101.2 ± 0.18 vs. 95.5 ± 0.20 μm) and diameter (51.2 ± 0.11 vs. 49.7 ± 0.07 μm), decreased VEGFA (14%) and unchanged capillary density. At 16 weeks, OIC presented increased in cardiomyocytes size (497.0±16.3 vs. 437.9±12.9µm2), and in capillary/cardiomyocyte density ratio (1.599±0.020 vs. 1.466±0.029 mm2) and downregulation of VEGFA (23%), VEGFR1 (27%), VEGFR2 (20%), and NRP1 (17%).
Conclusion:
Neonatal hyperoxia disrupts early LV coronary vascular development and induces long-term maladaptive remodeling. Early capillary rarefaction despite elevated VEGFA along with long-term cardiomyocytes hypertrophy and receptor downregulation suggests disrupt angiogenesis. These results reinforce the distinct alterations elicited by PT-related conditions and enhance the mechanistic understanding of increased susceptibility to cardiac diseases after PT.
More abstracts on this topic:
All Magnesium-Based Bioresorbable Scaffolds in patients with CAD: A Proportional Meta Analysis
Dar Areej, Abu Ajamieh Kamal, Abdullah Muhammad, Khalid Ghina, Kamel Mohammed, Dar Muhammad Shayaan
Perivascular Adipose Tissues: Developmental Origins and Early DifferentiationShadowens Alyssa, Rendon C. Javier, Demireva Elena, Watts Stephanie, Contreras Andres