Final ID: MP2673
Antibody-conjugated SIS3-loaded polymeric nanoparticles alleviate acute myocardial infarction- and pressure overload-induced cardiac fibrosis by targeting myofibroblasts
Abstract Body (Do not enter title and authors here): Background: Pathological cardiac fibrosis arising from acute/chronic myocardial injury drives ventricular remodeling, functional impairment, and elevated mortality through mechanisms lacking effective therapies. Central to fibrogenesis, myofibroblast activation via TGFβ1-Smad3 signaling necessitates targeted therapeutic strategies. We developed an antibody-conjugated nanoplatform delivering Smad3 inhibitor SIS3 specifically to activated fibroblasts.
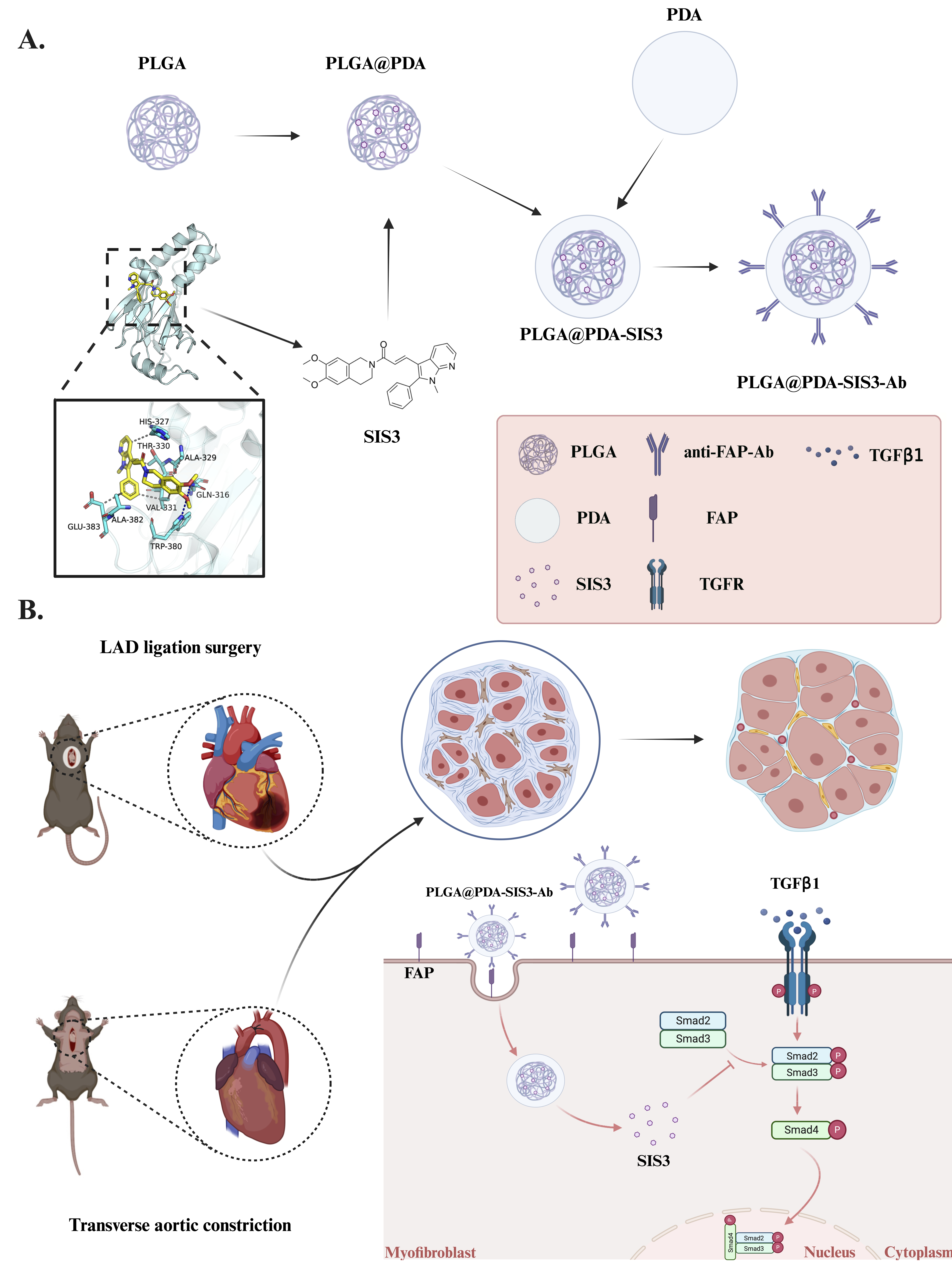
Methods: Anti-fibroblast activation protein (FAP)-functionalized nanoparticles (NPs-SIS3-Ab) were fabricated using PLGA@PDA cores via solvent evaporation. The morphological and biological characteristics of the nanoparticles were evaluated using transmission electron microscopy (TEM) and dynamic light scattering (DLS). In vitro targeting was assessed through confocal microscopy in TGFβ1-stimulated myofibroblasts. Therapeutic efficacy was evaluated in murine acute myocardial infarction (AMI) and transverse aortic constriction (TAC) models using in vivo imaging system,confocal microscopy, histopathology, transthoracic echocardiography and Western blotting.
Results: The NPs-SIS3-Ab nanoparticles demonstrated efficient SIS3 loading, antibody conjugation density, and colloidal stability. Anti-FAP antibody modification enabled specific targeting ability to myofibroblasts both in vitro and in vivo. NPs-SIS3-Ab significantly suppressed TGFβ1-Smad3 pathway activation of myofibroblast in vitro and furthermore, systemic intravenous delivery of NPs-SIS3-Ab markedly inhibited Smad3 phosphorylation, decreased cardiac fibrosis area and more importantly, restored cardiac function both in AMI (PBS VS NPs-SIS3 VS NPs-SIS3-Ab: 19.31 ± 4.43% VS 35.82 ± 1.93% VS 42.46 ± 0.97%, P <0.05-0.0001 ) and TAC (PBS VS NPs-SIS3 VS NPs-SIS3-Ab: 29.41 ± 2.08% VS 29.89 ± 2.97% VS 445.26 ± 3.02%, P <0.01) models without evident side effects.
Conclusion: This FAP-targeted nanoplatform achieves precision Smad3 inhibition, demonstrating dual efficacy against acute ischemic and chronic pressure-overload fibrosis while preserving systemic safety. Our findings establish a clinically translatable strategy for modulating pathological fibroblast activity in heart failure.
Methods: Anti-fibroblast activation protein (FAP)-functionalized nanoparticles (NPs-SIS3-Ab) were fabricated using PLGA@PDA cores via solvent evaporation. The morphological and biological characteristics of the nanoparticles were evaluated using transmission electron microscopy (TEM) and dynamic light scattering (DLS). In vitro targeting was assessed through confocal microscopy in TGFβ1-stimulated myofibroblasts. Therapeutic efficacy was evaluated in murine acute myocardial infarction (AMI) and transverse aortic constriction (TAC) models using in vivo imaging system,confocal microscopy, histopathology, transthoracic echocardiography and Western blotting.
Results: The NPs-SIS3-Ab nanoparticles demonstrated efficient SIS3 loading, antibody conjugation density, and colloidal stability. Anti-FAP antibody modification enabled specific targeting ability to myofibroblasts both in vitro and in vivo. NPs-SIS3-Ab significantly suppressed TGFβ1-Smad3 pathway activation of myofibroblast in vitro and furthermore, systemic intravenous delivery of NPs-SIS3-Ab markedly inhibited Smad3 phosphorylation, decreased cardiac fibrosis area and more importantly, restored cardiac function both in AMI (PBS VS NPs-SIS3 VS NPs-SIS3-Ab: 19.31 ± 4.43% VS 35.82 ± 1.93% VS 42.46 ± 0.97%, P <0.05-0.0001 ) and TAC (PBS VS NPs-SIS3 VS NPs-SIS3-Ab: 29.41 ± 2.08% VS 29.89 ± 2.97% VS 445.26 ± 3.02%, P <0.01) models without evident side effects.
Conclusion: This FAP-targeted nanoplatform achieves precision Smad3 inhibition, demonstrating dual efficacy against acute ischemic and chronic pressure-overload fibrosis while preserving systemic safety. Our findings establish a clinically translatable strategy for modulating pathological fibroblast activity in heart failure.
More abstracts on this topic:
Assessment of Adverse Left Ventricular Remodeling Following Ischemia Reperfusion Injury with SPECT Imaging Agent Targeting Fibroblast Activation Protein
Thorn Stephanie, Duncan James S, Spinale Francis, Luna Gutierrez Myrna, Ferro-flores Guillermina, Sinusas Albert, Porcaro Olivia, Burns Rachel, Zohora Fatema Tuj, Guerrera Nicole, Jang Sun-joo, Vermillion Billy, Lima Moroni, Liu Chi
Absence of standard modifiable risk factors (SMuRF-less) among 5002 Middle Eastern patients with atherosclerotic cardiovascular disease: (Interim analysis from the Jo-SMuRF Study)Aldalal'ah Mo'men, Hammoudeh Ayman, Hamza Ibrahem, Alqudah Mohammad, Khasawneh Hasan, Alomari Sawsan, Alomari Ahmad, H. Assaf Sarah, Zaqqa Ayah, Khatatbeh Moawiah