Final ID: MP2511
Finerenone and liver fibrosis assessed by fibrosis-4 (FIB-4) index in patients with heart failure and mildly reduced or preserved ejection fraction: Results from the FINEARTS-HF trial
Research Questions: What is the prognostic value of the FIB-4 index in patients with heart failure and mildly reduced or preserved ejection fraction (HFmrEF/HFpEF).
Methods: We analysed data from FINEARTS-HF, a phase 3, randomized, placebo-controlled trial evaluating the efficacy and safety of finerenone in patients with HFmrEF/HFpEF. The FIB-4 index was calculated using the formula: (age [years] × AST [U/L])/(platelets [109/L] × sqrt [ALT] [U/L]). Patients were grouped based on the risk of progressive fibrosis determined using the FIB-4 index: low (<1.30), indeterminate (1.30–2.67), and high (>2.67) risk (with a cutoff of 2.0 for those aged >65 years). Outcomes were examined using semiparametric proportional rates models for total events and Cox models for time-to-first-event data, stratified by region, and LVEF (<60%, ≥60%).
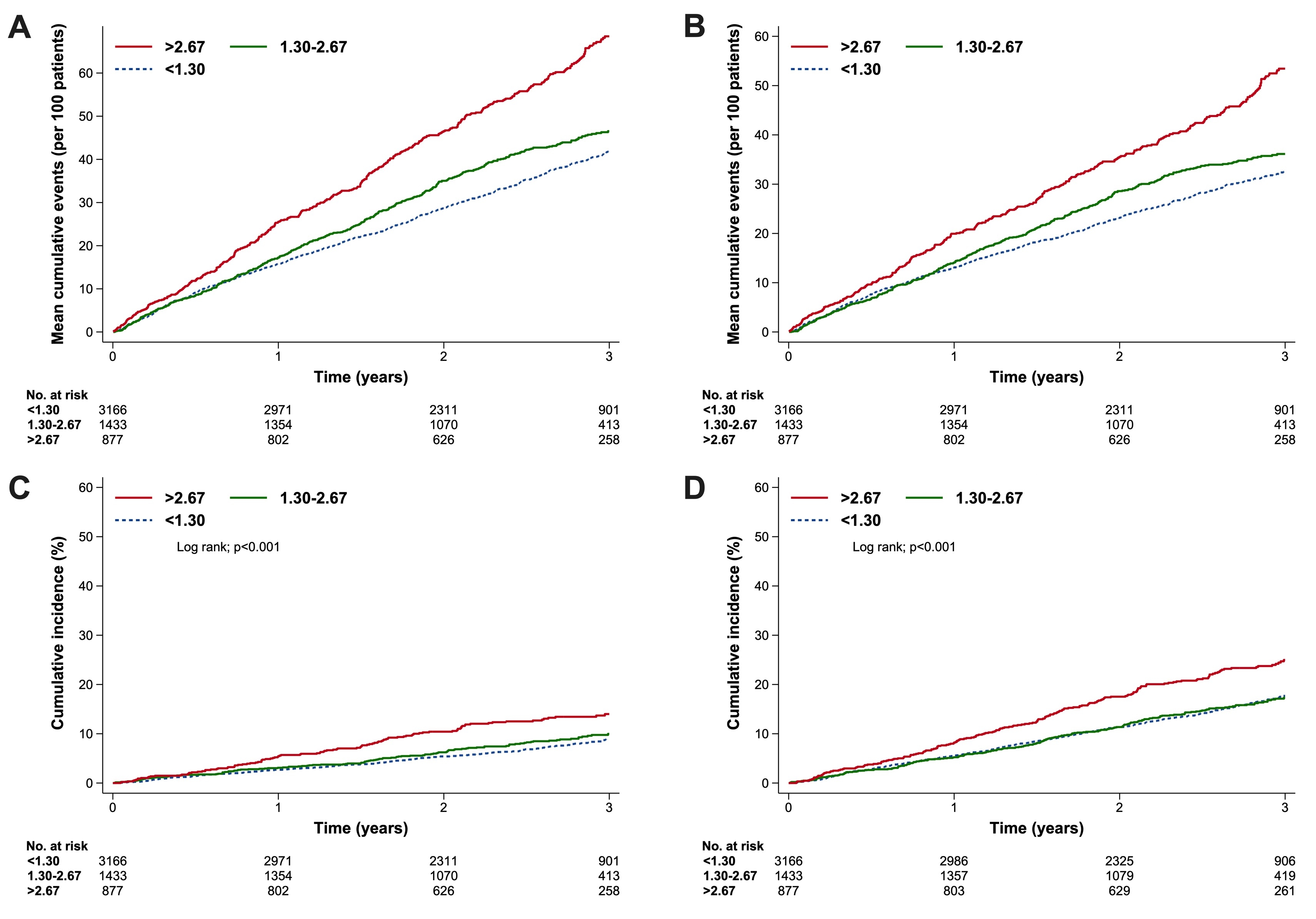
Results: Of 6001 randomised patients, 5476 (91.3%) had baseline FIB-4 index: 3166 (57.8%) were low risk, 1433 (26.2%) indeterminate, and 877 (16.0%) high risk. Compared with low risk of progressive fibrosis, patients with high risk were older, more often male and Asian, and more likely to have atrial fibrillation, prior stroke, higher N-terminal pro B-type natriuretic peptide (NT-proBNP), and worse kidney function. A higher FIB-4 index was associated with greater risk of the primary endpoint (cardiovascular death and total worsening heart failure events), its components, and similarly, all-cause death (Figure). Finerenone, compared with placebo, reduced the risk of the primary endpoint across all FIB-4 index groups: low risk: RR: 0.73 (95% CI: 0.61-0.88); indeterminate: 0.91 (95% CI: 0.69-1.19); high risk: 0.80 (95% CI: 0.61-1.07). The benefit of finerenone was not modified by FIB-4 index (Pinteraction = 0.38).
Conclusions: FIB-4, potentially reflecting liver fibrosis, independently predicts the risk of HF events in HFmrEF/HFpEF. Finerenone consistently reduced HF events across the low, indeterminate, and high FIB-4 index.
- Yang, Mingming ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Amarante, Flaviana ( Bayer AG , Berlin , Germany )
- De Sanctis, Yoriko ( Bayer AG , Berlin , Germany )
- Rohwedder, Katja ( Bayer AG , Berlin , Germany )
- Lam, Carolyn ( NATIONAL HEART CENTRE SINGAPORE , Singapore , Singapore )
- Senni, Michele ( ASST PAPA GIOVANNI XXIII , Bergamo , Italy )
- Shah, Sanjiv ( NORTHWESTERN UNIVERSITY , Chicago , Illinois , United States )
- Zannad, Faiez ( CVCT and Universite de Lorraine , Paris , France )
- Pitt, Bertram ( University of Michigan School , Ann Arbor , Michigan , United States )
- Vaduganathan, Muthiah ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Jhund, Pardeep ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Chimura, Misato ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Henderson, Alasdair David ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Talebi, Atefeh ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Desai, Akshay ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Kalayci, Arzu ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Sauer, Andrew ( Saint Lukes Mid America Heart Inst , Kansas City , Missouri , United States )
- Taub, Pam ( Division of Cardiovascular Medicine , San Diego , California , United States )
Meeting Info:
Session Info:
(Non-) Roid Rage: Novel Insights Into Non-Steroidal MRAs for HF
Monday, 11/10/2025 , 12:15PM - 01:25PM
Moderated Digital Poster Session
More abstracts on this topic:
Nishioka Norihiro, Kiguchi Takeyuki, Makino Yuto, Ninomiya Kouhei, Kamo Wataru, Kamada Tsuyoshi, Maeda Hideki, Iwami Taku
A Highly Selective and Orally Available HDAC6 Inhibitor, EKZ-102, Ameliorates Cardiac Dysfunction and Exercise Intolerance in Cardiometabolic HFpEFElbatreek Mahmoud, Goodchild Traci, Lefer David, Evans Lauren, Richardson Thomas, James Rebecca, Schroeder Frederick, Wang Jianhong, Luterman Jim, Gilbert Tonya, Fisher Richard
More abstracts from these authors:
Chimura Misato, Lam Carolyn, Senni Michele, Shah Sanjiv, Voors Adriaan, Zannad Faiez, Pitt Bertram, Vaduganathan Muthiah, Solomon Scott, Mcmurray John, Jhund Pardeep, Henderson Alasdair David, Claggett Brian, Desai Akshay, Mueller Katharina, Glasauer Andrea, Rohwedder Katja, Viswanathan Prabhakar
Efficacy of finerenone in patients with heart failure and mildly reduced or preserved ejection fraction: A prespecified analysis of heart rate in the FINEARTS-HF trialChimura Misato, Senni Michele, Zannad Faiez, Pitt Bertram, Vaduganathan Muthiah, Solomon Scott, Mcmurray John, Jhund Pardeep, Henderson Alasdair David, Claggett Brian, Desai Akshay, Lay-flurrie James, Scalise Andrea, Rohwedder Katja, Lam Carolyn