Final ID: MP791
Serum Transthyretin as a Screening Tool For Transthyretin Amyloid Cardiomyopathy
Abstract Body (Do not enter title and authors here): Abstract
Introduction
Diagnosing transthyretin amyloid cardiomyopathy (ATTR-CM) remains challenging due to overlapping clinical features with causes of heart failure. Current screening tools primarily detect advanced disease and often miss early or atypical presentations of ATTR-CM. Serum transthyretin (TTR) has emerged as a potential low-cost biomarker for broad ATTR-CM screening.
Aims
To evaluate whether serum TTR can facilitate screening for ATTR-CM in patients with suspected disease.
Methods
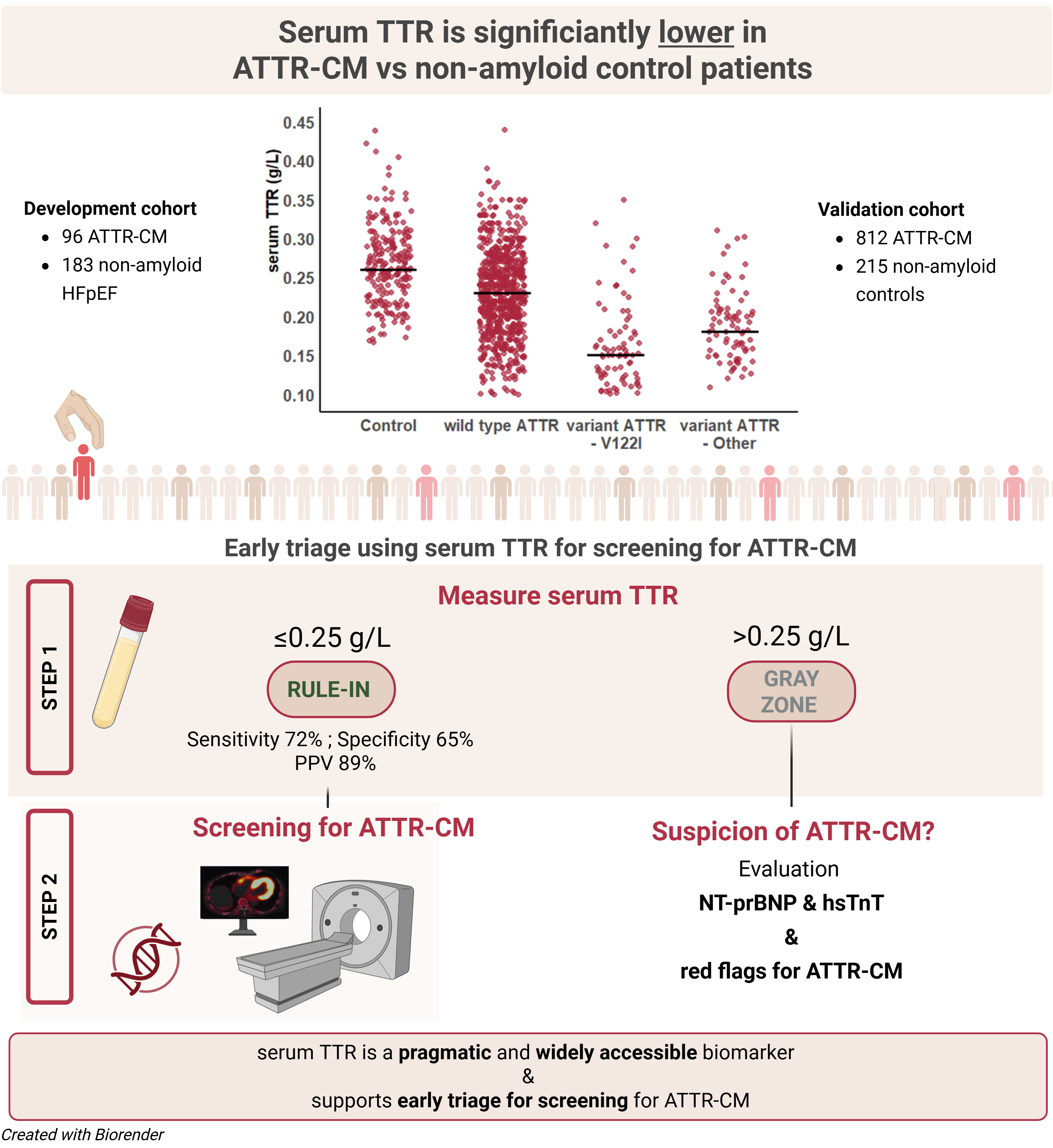
In this multicentre diagnostic study, serum TTR concentrations were compared between patients with ATTR-CM (n=96 in the development cohort; n=812 in the validation cohort) and non-amyloid controls (n=183 development cohort; n=215 validation cohort). The optimal cut-off value was determined using Youden’s index. Multivariable logistic regression was performed to assess the association between serum TTR and ATTR-CM, adjusted for clinical factors (e.g. Age, sex, NT-proBNP, hsTnT and echocardiographic parameters).
Results
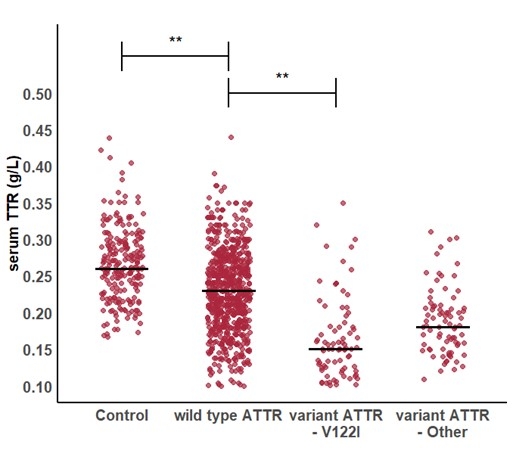
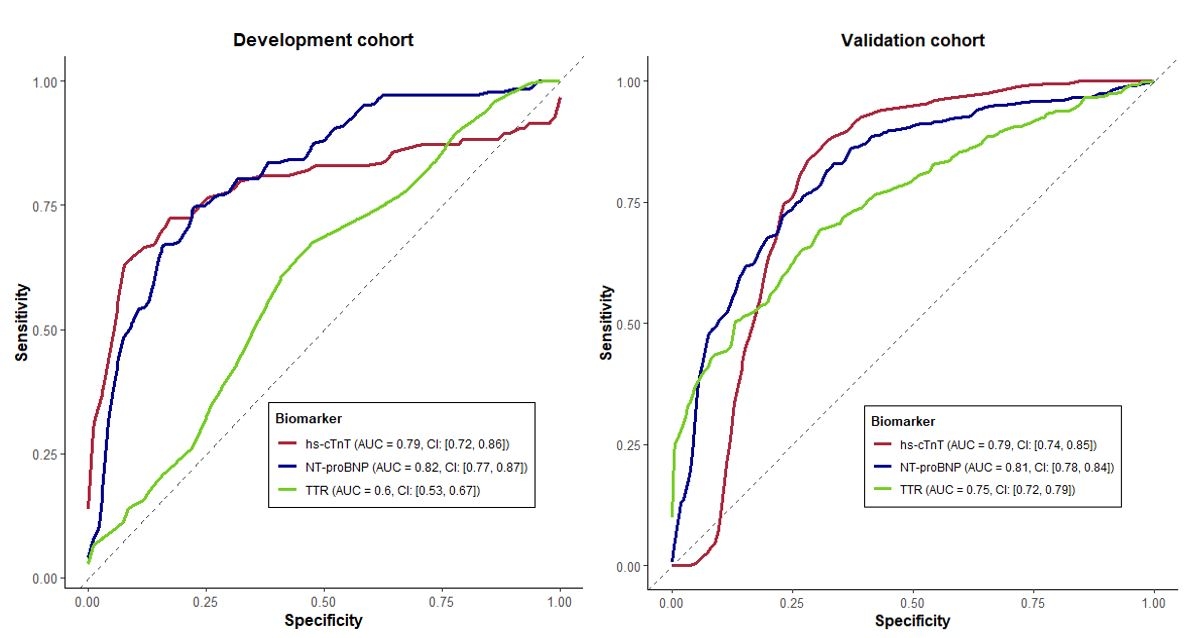
Serum TTR was significantly lower in ATTR-CM compared to controls (Figure 1; p<0.001), even after matching for age, sex and body mass index. Patients with variant ATTR-CM had significantly lower serum TTR concentration compared to those with wild-type ATTR-CM (0.16g/L – IQR [0.13, 0.20] vs 0.22g/L IQR [0.19, 0.26] respectively, p<0.001). Variant ATTR-CM patients with the p.(V142I) variant presented with the lowest serum TTR concentration (0.15g/L IQR [0.11, 0.18], Figure 1). The diagnostic performance of TTR alone was modest (AUC 0.60–0.75). A threshold of ≤0.25 g/L was identified as the optimal cut-off (Figure 2). In the validation cohort, a serum TTR level ≤0.25 g/L had 72% sensitivity and 65% specificity with a positive predictive value 89% for ATTR-CM. Multivariable regression confirmed serum TTR concentration as an independent predictor of ATTR-CM (adjusted OR 0.25 per 0.1 g/L increase, p<0.001).
Conclusion
A serum TTR concentration of ≤0.25 g/L is a strong indicator of underlying ATTR-CM in heart failure patients. Routine measurement of serum TTR could serve as a simple, accessible, and cost-effective initial screening tool to identify patients who should undergo diagnostic evaluation for ATTR-CM. Incorporating serum TTR into clinical pathways may facilitate earlier diagnosis and enable timely initiation of disease-modifying therapies.
Introduction
Diagnosing transthyretin amyloid cardiomyopathy (ATTR-CM) remains challenging due to overlapping clinical features with causes of heart failure. Current screening tools primarily detect advanced disease and often miss early or atypical presentations of ATTR-CM. Serum transthyretin (TTR) has emerged as a potential low-cost biomarker for broad ATTR-CM screening.
Aims
To evaluate whether serum TTR can facilitate screening for ATTR-CM in patients with suspected disease.
Methods
In this multicentre diagnostic study, serum TTR concentrations were compared between patients with ATTR-CM (n=96 in the development cohort; n=812 in the validation cohort) and non-amyloid controls (n=183 development cohort; n=215 validation cohort). The optimal cut-off value was determined using Youden’s index. Multivariable logistic regression was performed to assess the association between serum TTR and ATTR-CM, adjusted for clinical factors (e.g. Age, sex, NT-proBNP, hsTnT and echocardiographic parameters).
Results
Serum TTR was significantly lower in ATTR-CM compared to controls (Figure 1; p<0.001), even after matching for age, sex and body mass index. Patients with variant ATTR-CM had significantly lower serum TTR concentration compared to those with wild-type ATTR-CM (0.16g/L – IQR [0.13, 0.20] vs 0.22g/L IQR [0.19, 0.26] respectively, p<0.001). Variant ATTR-CM patients with the p.(V142I) variant presented with the lowest serum TTR concentration (0.15g/L IQR [0.11, 0.18], Figure 1). The diagnostic performance of TTR alone was modest (AUC 0.60–0.75). A threshold of ≤0.25 g/L was identified as the optimal cut-off (Figure 2). In the validation cohort, a serum TTR level ≤0.25 g/L had 72% sensitivity and 65% specificity with a positive predictive value 89% for ATTR-CM. Multivariable regression confirmed serum TTR concentration as an independent predictor of ATTR-CM (adjusted OR 0.25 per 0.1 g/L increase, p<0.001).
Conclusion
A serum TTR concentration of ≤0.25 g/L is a strong indicator of underlying ATTR-CM in heart failure patients. Routine measurement of serum TTR could serve as a simple, accessible, and cost-effective initial screening tool to identify patients who should undergo diagnostic evaluation for ATTR-CM. Incorporating serum TTR into clinical pathways may facilitate earlier diagnosis and enable timely initiation of disease-modifying therapies.
More abstracts on this topic:
Acoramidis Reduces All-Cause Mortality and Cardiovascular-Related Hospitalizations Through Month 42 in Transthyretin Amyloid Cardiomyopathy Across All Pre-specified Patient Subgroups
Stern Lily, Fine Nowell, Maurer Mathew, Grogan Martha, Ambardekar Amrut, Grodin Justin, Soman Prem, Garcia-pavia Pablo, Chen Chris, Siddhanti Suresh, Tamby Jean-francois, Fox Jonathan
Cardiac Troponin is Infrequently Tested in Emergency Department Patients with Suspected StrokeKolludra Kleona, Navi Babak, Merkler Alexander, Kamel Hooman, Liberman Ava