Final ID: MP2508
The Kaiser Permanente Cardiovascular Health Enhancement and Monitoring for Oncology (KP CHEMO) Study
Research Question: To delineate the contemporary incidence of cancer therapy-related cardiac dysfunction (CTRCD) in a real-world, diverse population.
Methods: We identified adults diagnosed with liquid or solid malignancy (excluding non-melanoma skin cancer) who received cardiotoxic cancer therapies (i.e., anthracyclines, human epidermal growth factor receptor 2 [HER2] inhibitors, immune checkpoint inhibitors [ICIs], tyrosine kinase inhibitors [TKIs]) within a large, integrated healthcare delivery system in Northern California from 2012-2022. The primary outcome was CTRCD, defined as either new-onset asymptomatic left ventricular dysfunction (i.e., a >10% absolute decrease in LVEF from ≥53% to <53%) or new-onset symptomatic heart failure, identified using a validated natural language processing algorithm applied to electronic health record data.
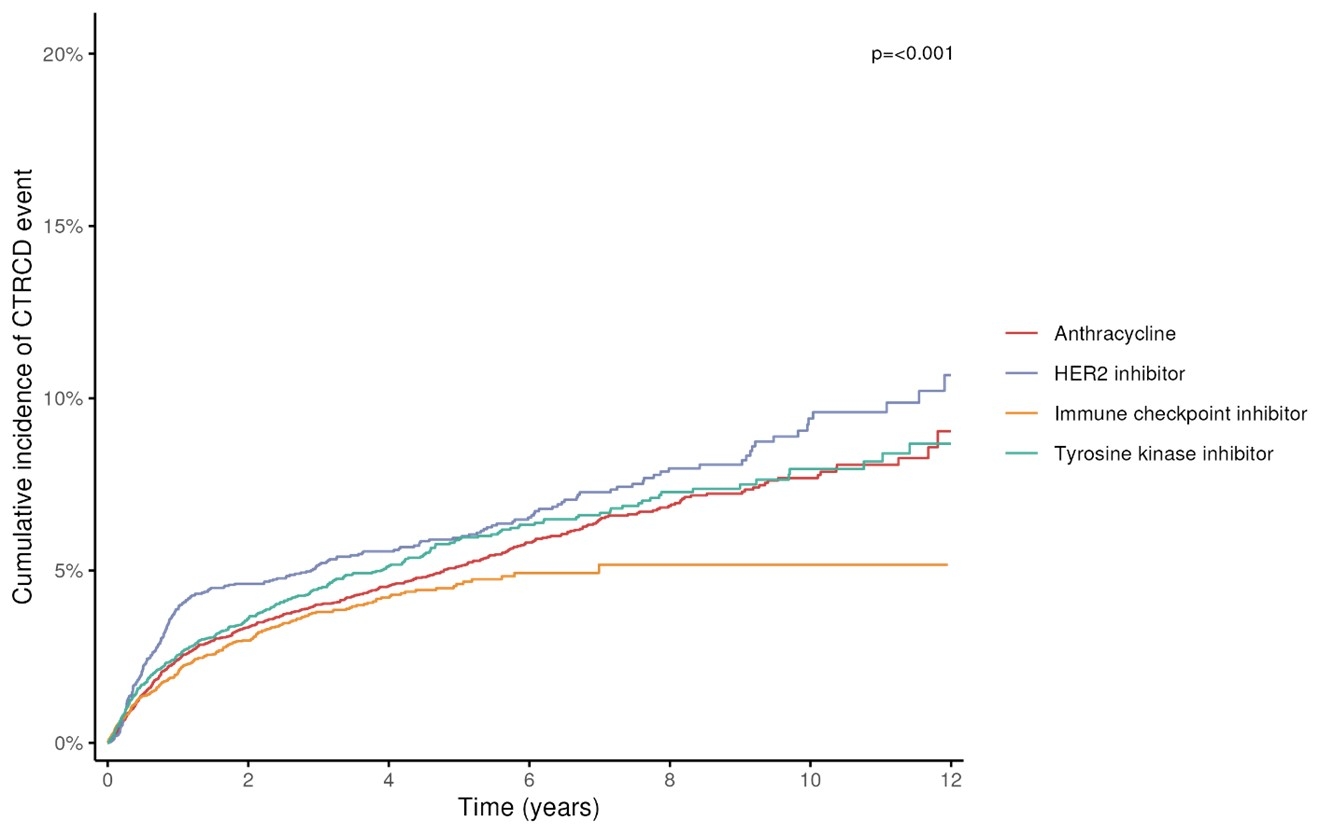
Results: Among 26,646 eligible patients, mean age was 62 ± 14 years, 64% were women, and 43% identified as a race/ethnicity other than non-Hispanic White. Breast (32%), lung (17%), and hematologic cancers (15%) were most common. Of the cohort, 38% received anthracyclines, 15% received HER2 inhibitors, 26% received ICIs, and 22% received TKIs. During a median of 2.0 [0.7-4.5] years of follow-up, the overall cumulative incidence of CTRCD was 8.4%, varying significantly by cancer therapy, with the highest for HER2 inhibitors (10.7%) and lowest for ICIs (5.2%) (P<0.001) (Figure). Early CTRCD (within 12 months) occurred at a higher rate (3.0 [2.8-3.3] per 100 person-years) than late CTRCD (1.1 [1.1-1.2] per 100 person-years), with nearly half of cases occurring in the first year of treatment.
Conclusions: In a large, contemporary cohort of cancer patients receiving cardiotoxic therapies, CTRCD affected nearly 1 in 10 patients, with many events occurring during the first year after treatment initiation. Validated prediction models are needed for short-term risk stratification, guiding targeted screening strategies, and earlier interventions to improve cardiovascular outcomes.
- Thadani, Samir ( Kaiser Permanente Northern California , Oakland , California , United States )
- Lopez, Alfredo ( Kaiser Permanente Northern California , Oakland , California , United States )
- Nugent, Joshua ( Kaiser Permanente Northern California , Oakland , California , United States )
- Ouyang, David ( Kaiser Permanente Northern California , Oakland , California , United States )
- Yen, Alberta ( Kaiser Permanente Northern California , Oakland , California , United States )
- Zaroff, Jonathan ( Kaiser Permanente Northern California , Oakland , California , United States )
- Ambrosy, Andrew ( Kaiser Permanente Northern California , Oakland , California , United States )
- Go, Alan ( Kaiser Permanente Northern California , Oakland , California , United States )
- Liu, Jane ( Kaiser Permanente Northern California , Oakland , California , United States )
- Garcia, Elisha ( Kaiser Permanente Northern California , Oakland , California , United States )
- Adatya, Sirtaz ( Kaiser Permanente Northern California , Oakland , California , United States )
- Bhatt, Ankeet ( Kaiser Permanente Northern California , Oakland , California , United States )
- Kwan, Marilyn ( Kaiser Permanente Northern California , Oakland , California , United States )
- Lin, Amy ( Kaiser Permanente Northern California , Oakland , California , United States )
- Liu, Raymond ( Kaiser Permanente Northern California , Oakland , California , United States )
Meeting Info:
Session Info:
Crossroads of Cancer and the Heart: Epidemiologic Insights in Cardio-Oncology
Monday, 11/10/2025 , 10:45AM - 12:00PM
Moderated Digital Poster Session
More abstracts on this topic:
Satish Vikyath, Pargaonkar Sumant, Slipczuk Leandro, Schenone Aldo, Maliha Maisha, Chi Kuan Yu, Sunil Kumar Sriram, Borkowski Pawel, Vyas Rhea, Rodriguez Szaszdi David Jose Javier, Kharawala Amrin, Seo Jiyoung
A Competency-Based Screening Echocardiography Curriculum Designed for Rural American Indian Community Health RepresentativesThoroughman Rose, Riley Alan, De Loizaga Sarah, Adams David, Beaton Andrea, Buonfiglio Samantha, Danforth Kristen, Masyuko Sarah, Miller Mccall, Yadava Mrinal
More abstracts from these authors:
Srikanth Kishan, Vasti Elena, Sandhu Alexander, Go Alan, Adatya Sirtaz, Bhatt Ankeet, Lee Keane, Parikh Rishi, Ambrosy Andrew, Tan Thida, Hamilton Steven, Vardeny Orly, Krishnaswami Ashok, Ku Ivy
Predicting Heart Failure with Improved Ejection Fraction Using Electronic Health Record-Based Models with Contemporary GDMTBhatt Ankeet, Go Alan, Parikh Rishi, Ambrosy Andrew, Tan Thida, Adatya Sirtaz, Lee Keane, Sandhu Alexander, Jana Svetlichnaya, Ku Ivy