Final ID: MP1927
Target Product Profile to Evaluate the Clinical Utility, Financial Impact, and Ethical Implications of an AI-Based HCM Detection Model
Abstract Body (Do not enter title and authors here): Hypertrophic cardiomyopathy (HCM) remains underdiagnosed despite effective therapies and accessible screening with electrocardiogram (ECG) and echocardiography. Multiple artificial intelligence (AI) tools show promise in identifying missed HCM cases; however, the path from a promising model to clinical impact remains unclear. Without clear performance thresholds and workflow integration parameters, health systems face uncertainty about which tool to adopt and how to responsibly deploy it.
We propose the use of Target Product Profiles (TPPs), an extension of the Fair, Useful, Reliable (AI) Models (FURM) Assessment framework, to define the minimum and ideal requirements for AI tools while incorporating resource, financial, and ethical considerations under real-world constraints. We developed a TPP to guide evaluation of an AI-augmented program for improving HCM diagnosis.
Using APLUS, a discrete-event simulation engine, we simulated an HCM screening workflow for 134,856 eligible patients within Stanford Health Care, a multi-hospital health system in California. The diagnostic workflow included primary care, echocardiography, triage, and HCM specialty clinic referral. We simulated multiple combinations of model sensitivity (0.5–0.975) and specificity (0.85–0.99), incorporating resource constraints (ex. HCM clinic capacity) and utility weights reflecting diagnostic delay, misdiagnosis, and mortality. Financial modeling included AI deployment costs and downstream care utilization. Ethical analysis was conducted through stakeholder interviews exploring issues such as perceived risks and benefits, equity, and patient consent.
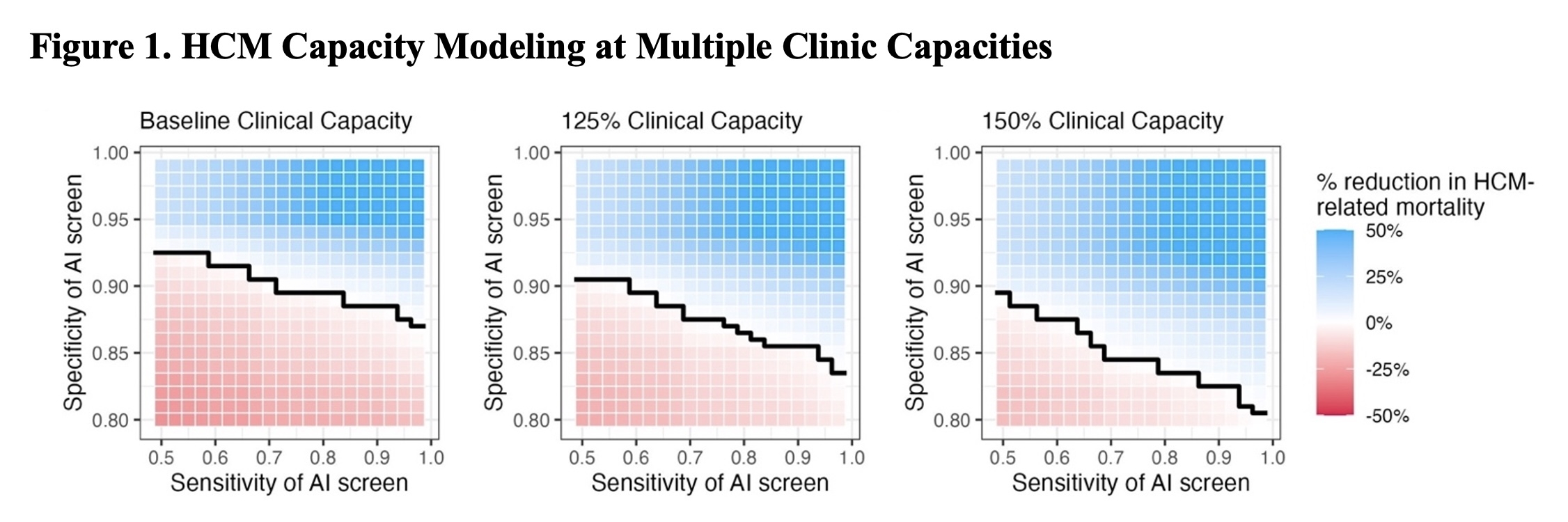
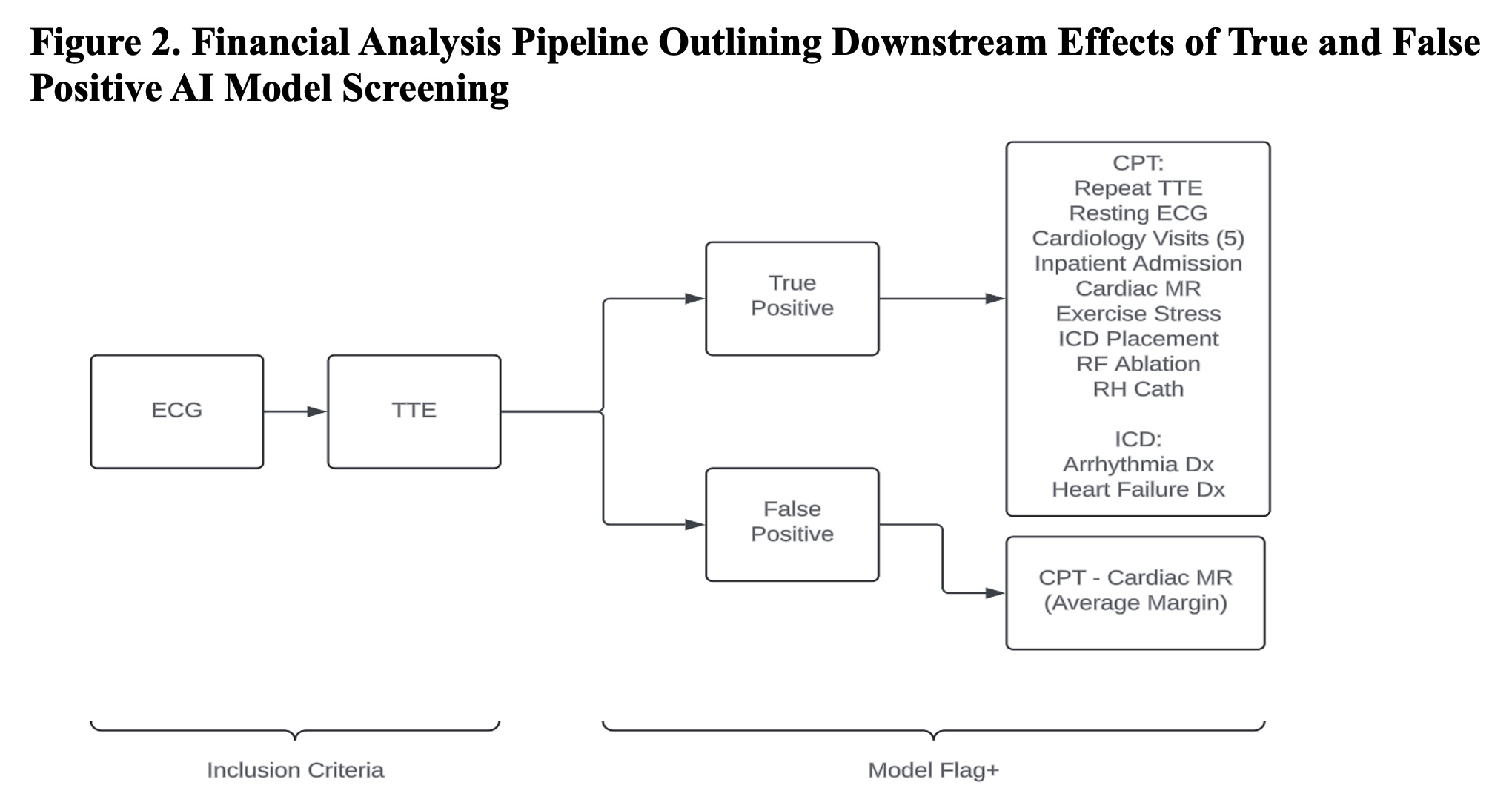
In our simulations, AI models with specificity ≥0.9 reduced HCM-related mortality using the proposed workflow, while lower specificity cutoffs overwhelmed referral capacity with false positive results (Figure 1). With a simulated 50% increase in HCM clinic capacity, a specificity of ≥0.85 was sufficient to achieve benefit. Financial models showed cost-effectiveness concentrated in true positive cases and a net positive effect for the hospital at low false-positive rates (Figure 2). Ethical review highlighted concerns and mitigation strategies around access disparities, patient anxiety from alerts, and subgroup representation.
For HCM, a TPP integrating workflow modeling, financial constraints, and ethical insights may help clarify necessary performance metrics in context—offering a roadmap for actionable, deployment-ready AI-augmented programs.
We propose the use of Target Product Profiles (TPPs), an extension of the Fair, Useful, Reliable (AI) Models (FURM) Assessment framework, to define the minimum and ideal requirements for AI tools while incorporating resource, financial, and ethical considerations under real-world constraints. We developed a TPP to guide evaluation of an AI-augmented program for improving HCM diagnosis.
Using APLUS, a discrete-event simulation engine, we simulated an HCM screening workflow for 134,856 eligible patients within Stanford Health Care, a multi-hospital health system in California. The diagnostic workflow included primary care, echocardiography, triage, and HCM specialty clinic referral. We simulated multiple combinations of model sensitivity (0.5–0.975) and specificity (0.85–0.99), incorporating resource constraints (ex. HCM clinic capacity) and utility weights reflecting diagnostic delay, misdiagnosis, and mortality. Financial modeling included AI deployment costs and downstream care utilization. Ethical analysis was conducted through stakeholder interviews exploring issues such as perceived risks and benefits, equity, and patient consent.
In our simulations, AI models with specificity ≥0.9 reduced HCM-related mortality using the proposed workflow, while lower specificity cutoffs overwhelmed referral capacity with false positive results (Figure 1). With a simulated 50% increase in HCM clinic capacity, a specificity of ≥0.85 was sufficient to achieve benefit. Financial models showed cost-effectiveness concentrated in true positive cases and a net positive effect for the hospital at low false-positive rates (Figure 2). Ethical review highlighted concerns and mitigation strategies around access disparities, patient anxiety from alerts, and subgroup representation.
For HCM, a TPP integrating workflow modeling, financial constraints, and ethical insights may help clarify necessary performance metrics in context—offering a roadmap for actionable, deployment-ready AI-augmented programs.
More abstracts on this topic:
AI and Quantum Sensors:
Realization of a Safe and Effective Unshielded Bedside Magnetocardiogram to Detect Ischemia in the Emergency Room
Realization of a Safe and Effective Unshielded Bedside Magnetocardiogram to Detect Ischemia in the Emergency Room
Iwata Geoffrey, Aschbacher Kirstin, John Sajiny, Tam Simon, Au-yeung Kit Yee, Contreras Johanna, Bhatt Deepak, Bander Jeffrey
A Novel EMR-Based Algorithm with the Virtual Echocardiography Screening Tool (VEST) to Screen Patients for Pulmonary Arterial HypertensionNarowska Gabriela, Anand Suneesh, Gangireddy Chethan, Enevoldsen John, Keane Martin, Edmundowicz Daniel, Forfia Paul, Vaidya Anjali