Final ID: Su4095
Monocyte-derived mitochondria-containing extracellular vesicles drive inflammation in heart failure
Abstract Body (Do not enter title and authors here): Introduction:
Sterile inflammation is increasingly recognized as a key contributor to heart failure (HF) progression, yet the lack of clearly defined molecular mechanisms has limited the development of targeted therapies. Among proposed triggers, mitochondrial damage-associated molecular patterns (MitoDAMPs)—immunogenic molecules released from injured mitochondria—are elevated in the circulation of HF patients. Since mitochondria are frequently exported within extracellular vesicles (EVs), we hypothesized that mitochondria-containing extracellular vesicles (EV-Mito), a previously unrecognized subclass of circulating EVs, may act as immune-activating mediators in HF.
Methods and Results:
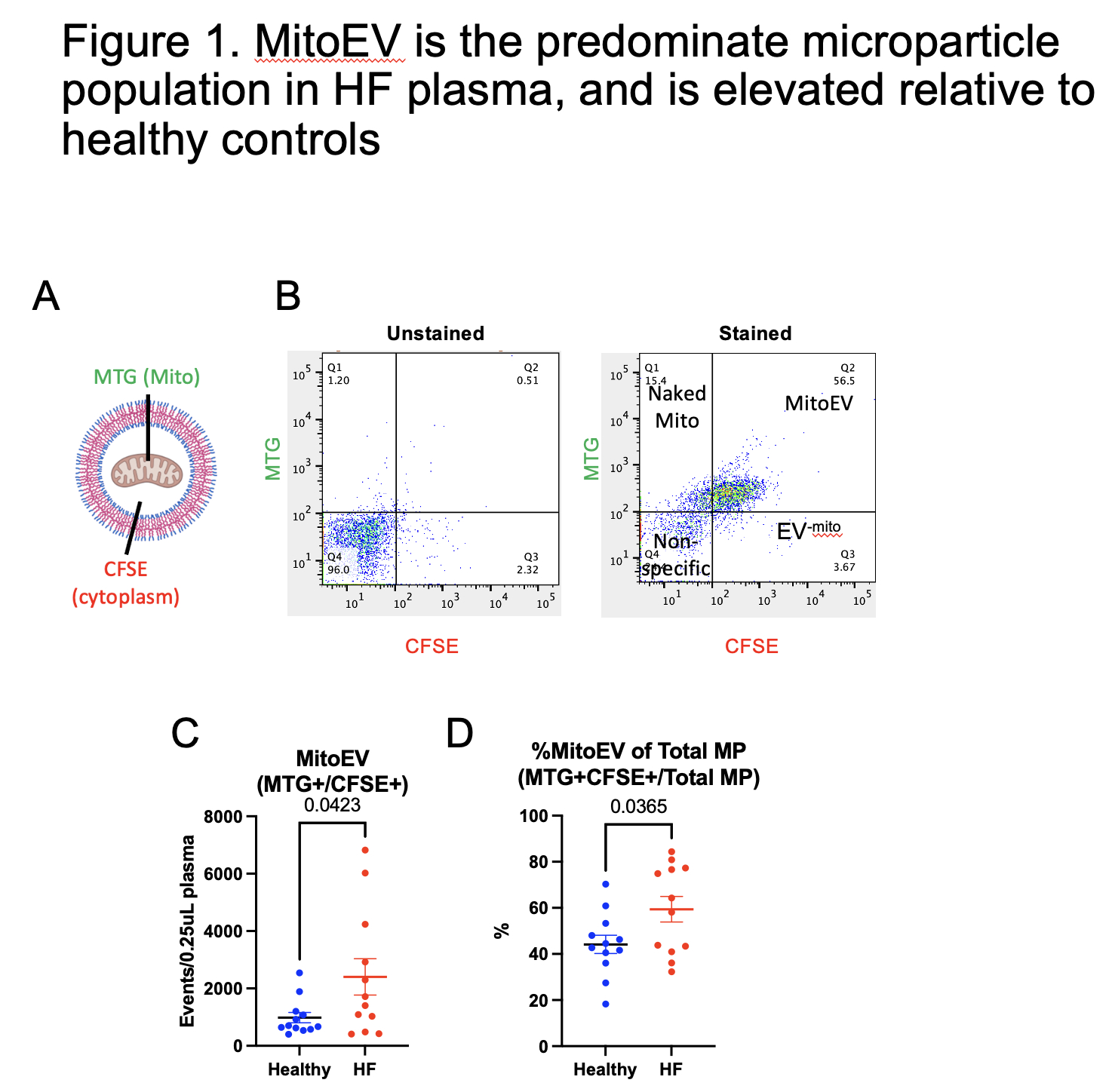
Plasma samples from patients with Stage D HFrEF (n=12) and healthy controls (n=12) were analyzed by flow cytometry using mitochondrial- and cytoplasmic-specific dyes. EV-Mito, defined as double-positive microparticles, were increased 3-fold in HF patients compared to controls (p = 0.0423) and accounted for over 70% of total plasma microparticles. EV-Mito isolated from HF plasma by size-exclusion chromatography followed by flow sorting induced robust IL1B and TNFα production in macrophages, confirming their immunogenicity. Surface marker profiling revealed CD14 enrichment, implicating monocytes as a primary source of EV-Mito in HF circulation.
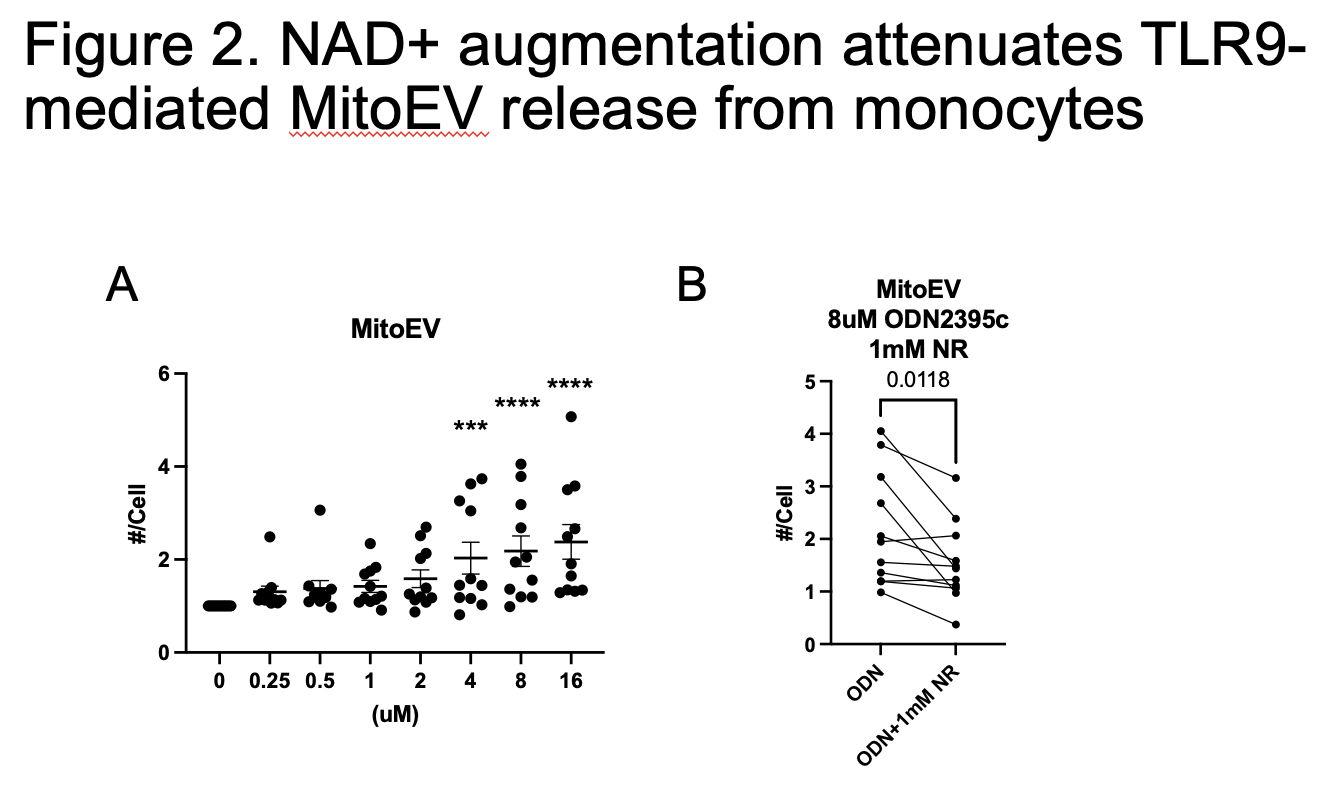
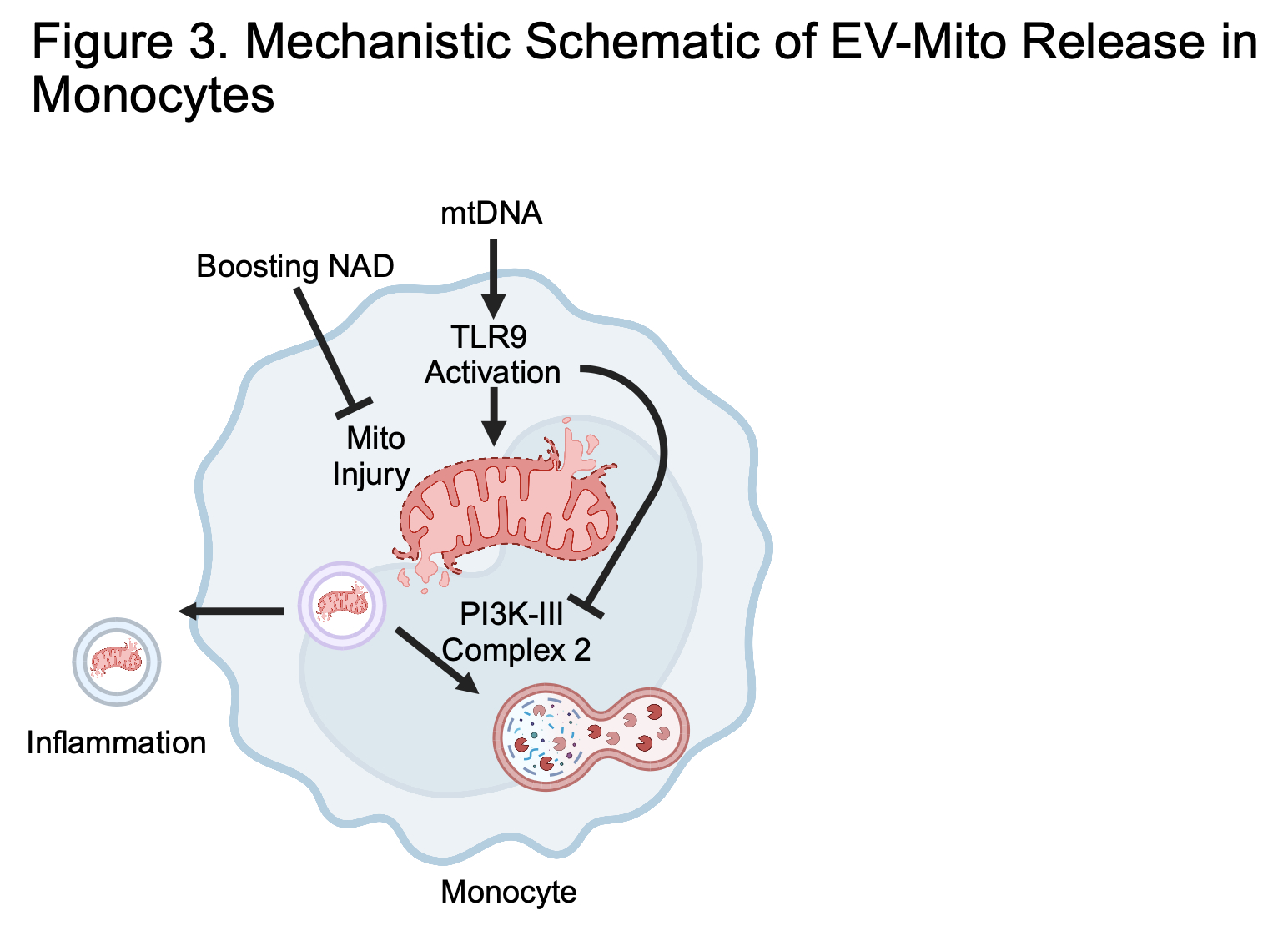
Mechanistically, mitochondrial DNA (mtDNA) activation of Toll-like receptor 9 (TLR9) in THP-1 monocytes induced mitochondrial dysfunction and impaired autophagy via disruption of the PI3K-III Complex II, resulting in EV-Mito release. Given the known role of NAD+ in preserving mitochondrial health, we tested nicotinamide riboside (an NAD+ precursor) supplementation in cultured monocytes and in HFrEF patients. While patient data are pending, NAD+ augmentation in primary monocytes preserved mitochondrial integrity and significantly reduced EV-Mito release by 30% (p = 0.0118).
Conclusion:
These findings identify EV-Mito as a mechanistically distinct and targetable mediator of inflammation in HF. By preventing their release, NAD+ augmentation offers a clinically feasible anti-inflammatory strategy with potential to improve outcomes in HF patients.
Sterile inflammation is increasingly recognized as a key contributor to heart failure (HF) progression, yet the lack of clearly defined molecular mechanisms has limited the development of targeted therapies. Among proposed triggers, mitochondrial damage-associated molecular patterns (MitoDAMPs)—immunogenic molecules released from injured mitochondria—are elevated in the circulation of HF patients. Since mitochondria are frequently exported within extracellular vesicles (EVs), we hypothesized that mitochondria-containing extracellular vesicles (EV-Mito), a previously unrecognized subclass of circulating EVs, may act as immune-activating mediators in HF.
Methods and Results:
Plasma samples from patients with Stage D HFrEF (n=12) and healthy controls (n=12) were analyzed by flow cytometry using mitochondrial- and cytoplasmic-specific dyes. EV-Mito, defined as double-positive microparticles, were increased 3-fold in HF patients compared to controls (p = 0.0423) and accounted for over 70% of total plasma microparticles. EV-Mito isolated from HF plasma by size-exclusion chromatography followed by flow sorting induced robust IL1B and TNFα production in macrophages, confirming their immunogenicity. Surface marker profiling revealed CD14 enrichment, implicating monocytes as a primary source of EV-Mito in HF circulation.
Mechanistically, mitochondrial DNA (mtDNA) activation of Toll-like receptor 9 (TLR9) in THP-1 monocytes induced mitochondrial dysfunction and impaired autophagy via disruption of the PI3K-III Complex II, resulting in EV-Mito release. Given the known role of NAD+ in preserving mitochondrial health, we tested nicotinamide riboside (an NAD+ precursor) supplementation in cultured monocytes and in HFrEF patients. While patient data are pending, NAD+ augmentation in primary monocytes preserved mitochondrial integrity and significantly reduced EV-Mito release by 30% (p = 0.0118).
Conclusion:
These findings identify EV-Mito as a mechanistically distinct and targetable mediator of inflammation in HF. By preventing their release, NAD+ augmentation offers a clinically feasible anti-inflammatory strategy with potential to improve outcomes in HF patients.
More abstracts on this topic:
Aminoacylase-1 Regulates Hepatic Mitochondrial Respiration and Whole-Body Metabolism
Guan Yuntian, Banks Alexander, Gerszten Robert, Benson Mark, Jonas Zachary, Wang Alissa, Tendoh Foje-geh, Manish Mahesh, Shah Radhe, Hofmann Alissa, Shen Dongxiao, Cortopassi Marissa
6-Nitrodopamine potentiates the positive chronotopic and inotropic effect induced by noradrenaline in the rat isolated heartLima Antonio, Sobanski Joao Fernando, Antunes Edson, De Nucci Gilberto