Final ID: We054
Mitochondria-containing Extracellular Vesicles Mediate Heart Failure Sterile Inflammation
Abstract Body: Introduction: Sterile inflammation has long been postulated to exacerbate heart failure (HF); however, a lack of viable biological targets and thorough understanding of the underlying mechanism posed formidable challenges in the development of novel therapeutic interventions.
Hypothesis: Given proinflammatory mitochondrial DNA (mtDNA) are known to be elevated in HF plasma; and that cells release mitochondrial components in extracellular vesicles, we hypothesize that proinflammatory circulating Mitochondria-containing Extracellular Vesicle (MitoEV) is a mediator of HF sterile inflammation.
Methods/Results: Using plasma samples of 12 Stage-D HFrEF and 12 healthy participants, plasma microparticles (MP) are co-stained with mitochondrial- and cytoplasmic-specific markers then quantified by volume-metric flow analysis. We found that there are significantly more mitochondrial-cytoplasmic double-positive MP (designated MitoEV, accounts for >70% of total MP) in HF relative to control. Next, using size-exclusion chromatography, we found MitoEV fractions are significantly more potent than soluble protein fractions to induce cytokine production in macrophage, implicating MitoEV as the source of HF plasma immunogenicity. Surface marker analysis by flow suggests that CD14+ monocytes is a major source of HF plasma MitoEV. Using THP-1 monocytes, we found that TLR9 activation by mtDNA results in injured mitochondria to be released as MitoEV (rather than removed by mitophagy), a pathway negatively regulated by beclin-1. Finally, boosting NAD by nicotinamide riboside preserves mitochondrial dysfunction and significantly reduces MitoEV release in primary monocytes.
Conclusion: Our findings implicate circulating MitoEV released by monocytes upon mtDNA activation as a pivotal mediator of sterile inflammation in HF, demonstrating a promising avenue for potential therapeutic targeting. The study also provides a potential cellular mechanism to the anti-inflammatory effect of boosting NAD.
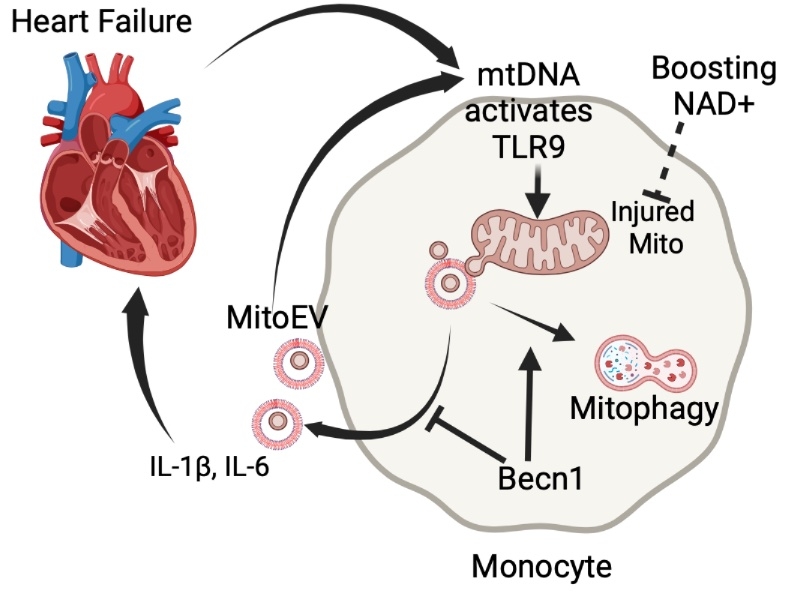
Model: In HF circulation, mtDNA activates TLR9 of monocytes, injuring mitochondria, which are released as MitoEV (containing mtDNA) to amplify and propagate inflammation in an autocrine manner. Becn1, a key mediator of mitophagy, negatively regulates MitoEV release. Activated monocytes also release cytokines to further injure the heart, resulting in a vicious cycle. Boosting NAD protects against mtDNA-induced mitochondrial dysfunction, attenuates the MitoEV release, and ameliorates inflammation.
Hypothesis: Given proinflammatory mitochondrial DNA (mtDNA) are known to be elevated in HF plasma; and that cells release mitochondrial components in extracellular vesicles, we hypothesize that proinflammatory circulating Mitochondria-containing Extracellular Vesicle (MitoEV) is a mediator of HF sterile inflammation.
Methods/Results: Using plasma samples of 12 Stage-D HFrEF and 12 healthy participants, plasma microparticles (MP) are co-stained with mitochondrial- and cytoplasmic-specific markers then quantified by volume-metric flow analysis. We found that there are significantly more mitochondrial-cytoplasmic double-positive MP (designated MitoEV, accounts for >70% of total MP) in HF relative to control. Next, using size-exclusion chromatography, we found MitoEV fractions are significantly more potent than soluble protein fractions to induce cytokine production in macrophage, implicating MitoEV as the source of HF plasma immunogenicity. Surface marker analysis by flow suggests that CD14+ monocytes is a major source of HF plasma MitoEV. Using THP-1 monocytes, we found that TLR9 activation by mtDNA results in injured mitochondria to be released as MitoEV (rather than removed by mitophagy), a pathway negatively regulated by beclin-1. Finally, boosting NAD by nicotinamide riboside preserves mitochondrial dysfunction and significantly reduces MitoEV release in primary monocytes.
Conclusion: Our findings implicate circulating MitoEV released by monocytes upon mtDNA activation as a pivotal mediator of sterile inflammation in HF, demonstrating a promising avenue for potential therapeutic targeting. The study also provides a potential cellular mechanism to the anti-inflammatory effect of boosting NAD.
Model: In HF circulation, mtDNA activates TLR9 of monocytes, injuring mitochondria, which are released as MitoEV (containing mtDNA) to amplify and propagate inflammation in an autocrine manner. Becn1, a key mediator of mitophagy, negatively regulates MitoEV release. Activated monocytes also release cytokines to further injure the heart, resulting in a vicious cycle. Boosting NAD protects against mtDNA-induced mitochondrial dysfunction, attenuates the MitoEV release, and ameliorates inflammation.
More abstracts on this topic:
A Growing Burden of Electronic Medical Record Messages in ACHD Care
Dailey Schwartz Andrew, Alegria Jorge
A Simple One-Item Nursing Falls Assessment Predicts Outcomes For Patients With Stage D Heart Failure Undergoing Surgical Advanced TherapiesSalvador Vincent, Perez Jaime Abraham, Hudec Paige, Gorodeski Eiran, Oneill Thomas