Final ID: Su4017
Therapeutic Targeting of Notch Signaling Rescues p.S13F-induced Desmin Cardiomyopathy
Abstract Body (Do not enter title and authors here): Background: Pathogenic DES mutations cause desmin cardiomyopathy (DesCM), characterized by desmin aggregation and intercalated disc dysfunction. Current therapeutic options for desmin cardiomyopathy remain limited, and the underlying pathogenic mechanisms are incompletely understood.
Methods: We identified a pathogenic DES-p.S13F mutation co-segregating with DesCM in a familial cohort. Des-p.S13F knock-in mice, des-S13F mutant zebrafish, and S13F-expressing neonatal mouse ventricular myocytes (NMVMs) were engineered to construct pathogenic desmin cardiomyopathy models based on the identified human mutation, enabling in vivo and in vitro investigation.Single-cell RNA sequencing of mouse hearts and bulk RNA sequencing of zebrafish hearts identified dysregulated signaling pathways in mutants. Based on these findings, Notch signaling was inhibited by DAPT or activated by Jagged1/melatonin in vivo and vitro to determine its protective role in DesCM caused by the S13F mutation.
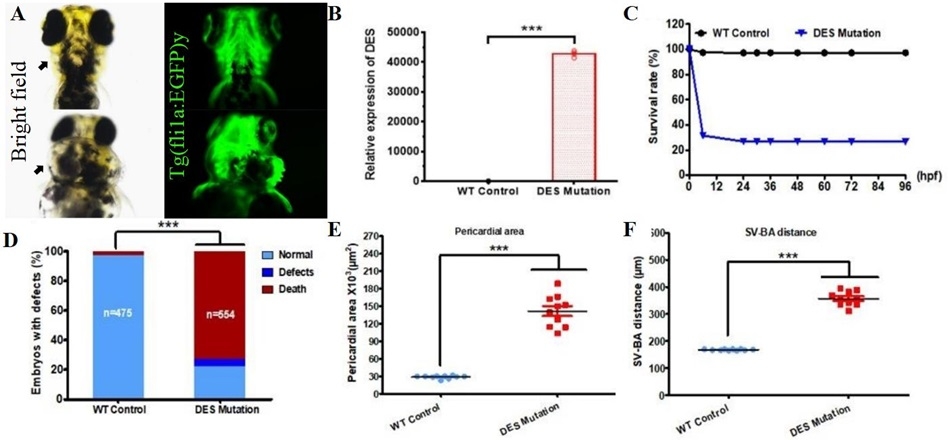
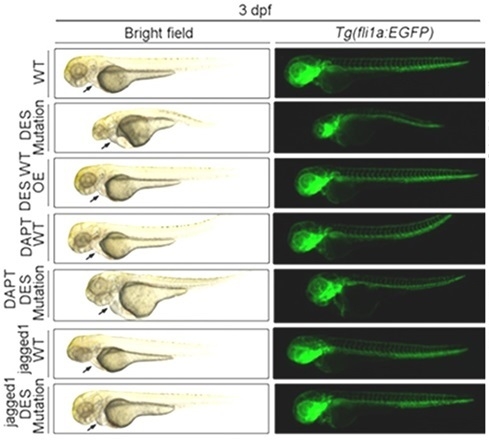
Results: Of the 69 family members screened for the DES-p.S13F mutation, 14 of them were positive, 6 were obligate carriers. Whole-exome sequencing of a DesCM family identified a pathogenic S13F mutation segregating. Des-p.S13F knock-in mice developed heart failure, exhibited increased desmin aggregation, and displayed reduced connexin 43 expression. des-S13F mutant zebrafish displayed pericardial edema, bradycardia, impaired cardiac regeneration, and vascular congestion. Single-cell RNA sequencing and bulk RNA-seq of mouse and zebrafish hearts revealed significant downregulation of the Notch signaling pathway in mutants. DAPT-mediated Notch inhibition exacerbated cardiac dysfunction, desmin aggregation, and connexin 43 reduction in mice with worsened pericardial edema in zebrafish. Conversely, Jagged1/melatonin-activated Notch signaling rescued these pathologies.
Conclusion: In summary, our findings demonstrate that impaired Notch signaling dysregulates desmin homeostasis and intercellular communication, driving cardiomyocyte dysfunction in p.S13F-induced DesCM, and suggest therapeutic modulation of Notch signaling as a novel interventional target.
Methods: We identified a pathogenic DES-p.S13F mutation co-segregating with DesCM in a familial cohort. Des-p.S13F knock-in mice, des-S13F mutant zebrafish, and S13F-expressing neonatal mouse ventricular myocytes (NMVMs) were engineered to construct pathogenic desmin cardiomyopathy models based on the identified human mutation, enabling in vivo and in vitro investigation.Single-cell RNA sequencing of mouse hearts and bulk RNA sequencing of zebrafish hearts identified dysregulated signaling pathways in mutants. Based on these findings, Notch signaling was inhibited by DAPT or activated by Jagged1/melatonin in vivo and vitro to determine its protective role in DesCM caused by the S13F mutation.
Results: Of the 69 family members screened for the DES-p.S13F mutation, 14 of them were positive, 6 were obligate carriers. Whole-exome sequencing of a DesCM family identified a pathogenic S13F mutation segregating. Des-p.S13F knock-in mice developed heart failure, exhibited increased desmin aggregation, and displayed reduced connexin 43 expression. des-S13F mutant zebrafish displayed pericardial edema, bradycardia, impaired cardiac regeneration, and vascular congestion. Single-cell RNA sequencing and bulk RNA-seq of mouse and zebrafish hearts revealed significant downregulation of the Notch signaling pathway in mutants. DAPT-mediated Notch inhibition exacerbated cardiac dysfunction, desmin aggregation, and connexin 43 reduction in mice with worsened pericardial edema in zebrafish. Conversely, Jagged1/melatonin-activated Notch signaling rescued these pathologies.
Conclusion: In summary, our findings demonstrate that impaired Notch signaling dysregulates desmin homeostasis and intercellular communication, driving cardiomyocyte dysfunction in p.S13F-induced DesCM, and suggest therapeutic modulation of Notch signaling as a novel interventional target.
More abstracts on this topic:
A Case of Clozapine-Induced Myocarditis: An Under-described Side Effect
Ibrahim Rand, Clearo Kellie
A Case of Dilated Cardiomyopathy and Systemic Thromboembolism in a Young Patient on Testosterone Replacement TherapySabri Muhammad, Ijaz Naila, Nadeem Ramsha, Checchio Lucy, Riaz Faiza