Final ID: Su4004
Spatial Organizations of Heterochromatin Underlie Cardiomyocyte Nuclear Structural Integrity Against Mechanical Stress
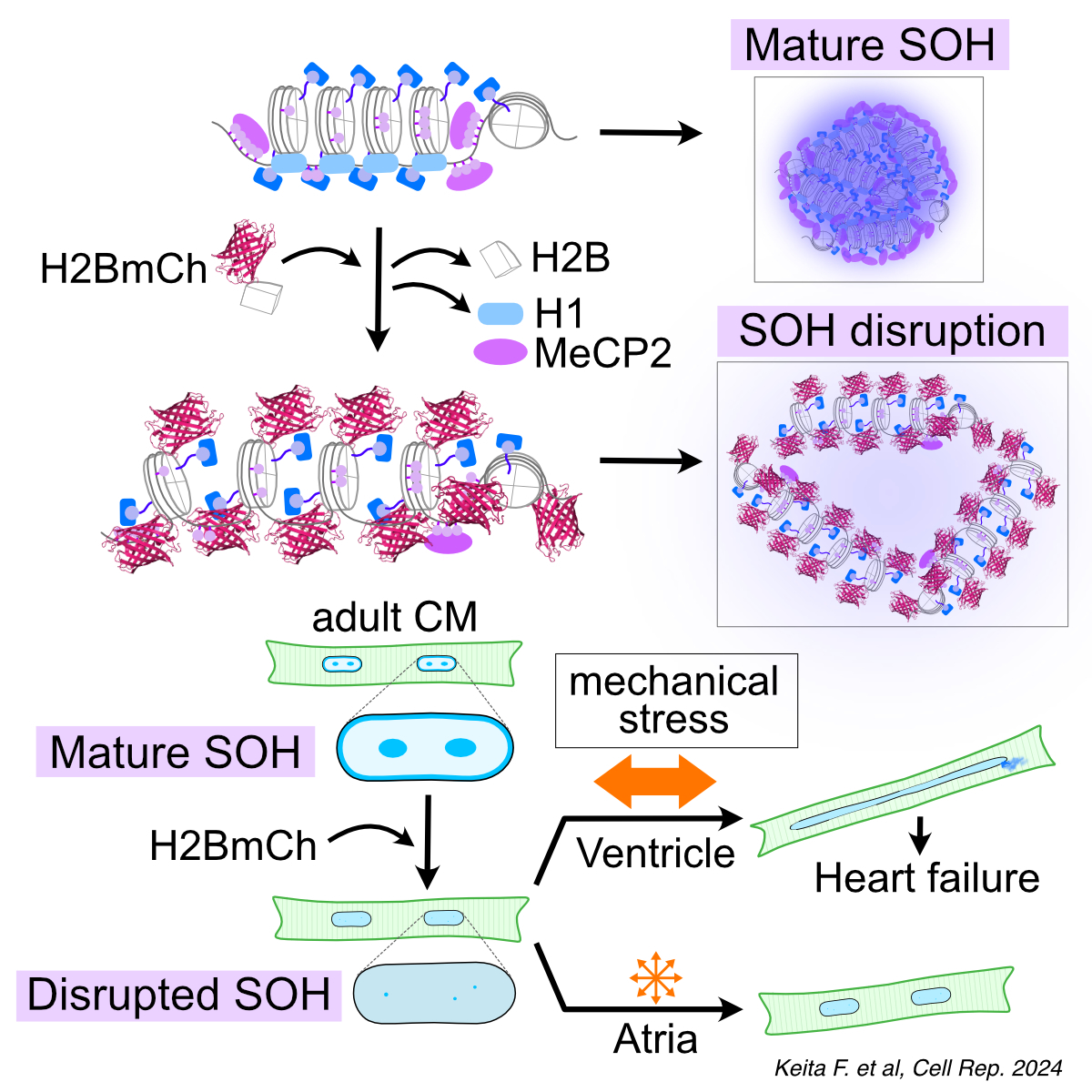
Abstract Body (Do not enter title and authors here): Introduction: Cardiomyocyte (CM) nuclei endure relentless contraction cycles while preserving nuclear architecture and transcriptional fidelity. In mice engineered for ErbB4-driven CM proliferation—with H2B-mCherry as a CM nuclei marker—we unexpectedly observed heterochromatin loss, extreme nuclear elongation, envelope rupture, and fatal heart failure, implicating a physical role for heterochromatin in nuclear integrity.
Hypothesis: We propose that spatial organizations of heterochromatin (SOH)—a peripheral layer plus chromocenters visible by microscopy—serve as a structural platform essential for CM nuclear integrity.
Methods: We generated mice with CM-specific overexpression (OE) of H2B-mCherry, H2B alone, or NLS-mCherry using Troponin T–Cre (constitutive) and MerCreMer (inducible). Cardiac function was monitored by echocardiography. Nuclear morphology was examined by confocal and electron microscopy; stiffness by atomic force microscopy. Hi-C and ATAC-seq profiled chromatin topology and accessibility; RNA-seq assessed transcription. Western blots quantified histone composition. Statistics: two-tailed t-tests or ANOVA (α=0.05).
Results: Constitutive H2B-mCherry OE—but not H2B or NLS-mCherry—induced progressive CM nuclear elongation and lethal cardiomyopathy. In adult-inducible OE, SOH disappeared within two weeks–preceding any change in nuclear shape. CM nuclei then softened (a ~50% drop in Young’s modulus from 3.2→1.6kPa, p<0.001), elongated over two-fold, and ~23% ruptured, releasing DNA that activated cGAS/STING (cGAS ↑18.3-fold, p<0.01), driving inflammation and fibrosis. Hi-C revealed blurred TAD insulation, while ATAC-seq/RNA-seq were unchanged, indicating that SOH disruption compromises mechanics rather than gene expression. Westerns demonstrated H2B-mCherry incorporation displaces endogenous H2B and, via mCherry steric hindrance, dislodges histone H1 (–60%, p<0.001), loosening chromatin compaction, and disperses LLPS factors (e.g., MeCP2), abolishing SOH. Aged hearts recapitulated SOH dissipation, H1 reduction, and nuclear deformation, linking heterochromatin disruption to age-related cardiomyopathies.
Conclusion: Our data identify SOH as critical mechanical support for CM nuclei; their disruption softens nuclei, precipitates deformation and inflammation, and culminates in heart failure, highlighting heterochromatin maintenance as a novel therapeutic target in age-related cardiomyopathies.
Hypothesis: We propose that spatial organizations of heterochromatin (SOH)—a peripheral layer plus chromocenters visible by microscopy—serve as a structural platform essential for CM nuclear integrity.
Methods: We generated mice with CM-specific overexpression (OE) of H2B-mCherry, H2B alone, or NLS-mCherry using Troponin T–Cre (constitutive) and MerCreMer (inducible). Cardiac function was monitored by echocardiography. Nuclear morphology was examined by confocal and electron microscopy; stiffness by atomic force microscopy. Hi-C and ATAC-seq profiled chromatin topology and accessibility; RNA-seq assessed transcription. Western blots quantified histone composition. Statistics: two-tailed t-tests or ANOVA (α=0.05).
Results: Constitutive H2B-mCherry OE—but not H2B or NLS-mCherry—induced progressive CM nuclear elongation and lethal cardiomyopathy. In adult-inducible OE, SOH disappeared within two weeks–preceding any change in nuclear shape. CM nuclei then softened (a ~50% drop in Young’s modulus from 3.2→1.6kPa, p<0.001), elongated over two-fold, and ~23% ruptured, releasing DNA that activated cGAS/STING (cGAS ↑18.3-fold, p<0.01), driving inflammation and fibrosis. Hi-C revealed blurred TAD insulation, while ATAC-seq/RNA-seq were unchanged, indicating that SOH disruption compromises mechanics rather than gene expression. Westerns demonstrated H2B-mCherry incorporation displaces endogenous H2B and, via mCherry steric hindrance, dislodges histone H1 (–60%, p<0.001), loosening chromatin compaction, and disperses LLPS factors (e.g., MeCP2), abolishing SOH. Aged hearts recapitulated SOH dissipation, H1 reduction, and nuclear deformation, linking heterochromatin disruption to age-related cardiomyopathies.
Conclusion: Our data identify SOH as critical mechanical support for CM nuclei; their disruption softens nuclei, precipitates deformation and inflammation, and culminates in heart failure, highlighting heterochromatin maintenance as a novel therapeutic target in age-related cardiomyopathies.
More abstracts on this topic:
Amyloid Pathway Markers and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis
Sharma Gaurav, Gutta Durga Prasad, Javid Saman, Haider Salar, Lanka Nidhi, Mishra Tushar
Cardiomyocyte-specific PLCe1 knock-down attenuates Angiotensin II-induced cardiac hypertrophy.Ni Kareemah, Rigaud Vagner, Vigneault Patrick, Xiao Chunyang, Klattenhoff Carla, Ellinor Patrick