Final ID: MDP1357
Empagliflozin Inhibits the Mobilization of CCR2+ Monocyte-Derived Macrophages by Reducing CCL2 Expression in Fibroblasts and Slows the Progression of Heart Failure After Pressure Overload.
Abstract Body (Do not enter title and authors here): The positive effects of sodium glucose co-transporter-2 (SGLT2) inhibitors on reducing cardiovascular mortality and hospitalizations due to heart failure have been well established. Although numerous theories propose explanations for the cardioprotective actions of SGLT2 inhibitors, these hypotheses primarily rely on initial findings and require further validation. Studies have shown that immune cells, particularly macrophages marked by C-C chemokine receptor type 2 (CCR2), contribute to the progression of heart failure by infiltrating the myocardium and inducing detrimental structural changes following myocardial infarction or pressure overload.
This study aims to investigate whether SGLT2 inhibitors can mitigate heart damage by inhibiting the migration of CCR2+ monocyte-derived macrophages.
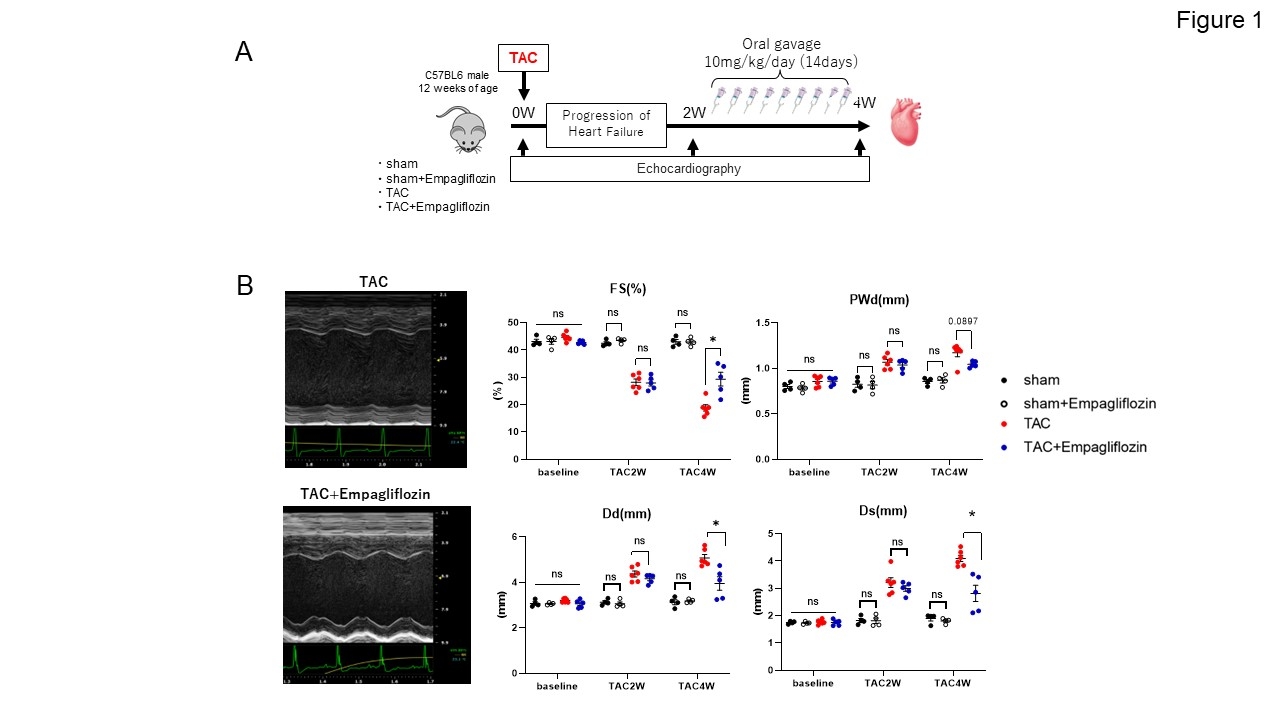
In this experiment, male B6 mice were subjected to transverse aortic constriction (TAC) to induce heart failure. Two weeks post-TAC, with heart function confirmed as equal across all groups by echocardiography, one group began receiving daily oral doses of empagliflozin, while another served as the control. At four weeks post-TAC, cardiac function was reassessed. The animals were then euthanized, and their hearts were analyzed using flow cytometry, staining, and qPCR to evaluate heart failure and the involvement of immune cells. Additionally, isolated cardiac fibroblasts were subjected to cyclic stretching to mimic post-TAC mechanical stress.
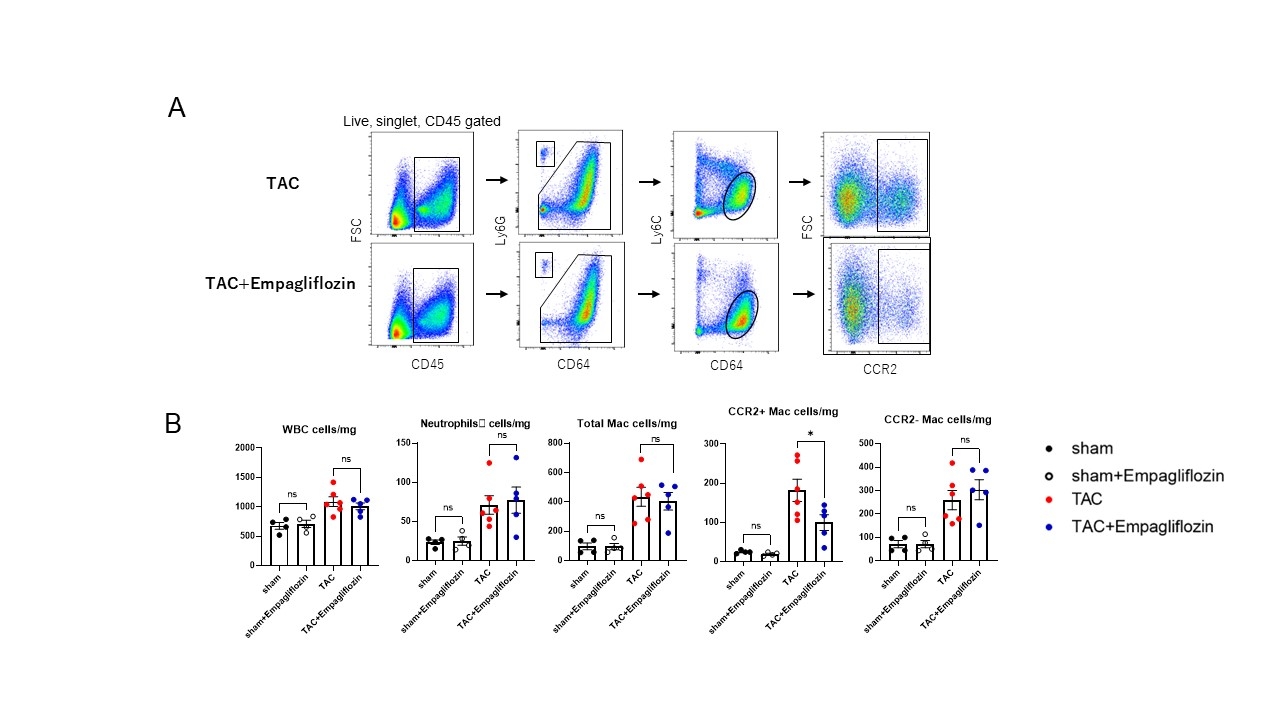
Treatment with empagliflozin resulted in improved cardiac function and reduced congestion in the heart and lungs, indicating alleviation of heart failure. The treatment notably decreased myocardial fibrosis, evident from Picrosirius-red staining and reduced expression of fibrosis-related mRNA. Importantly, empagliflozin specifically reduced the number of CCR2-positive macrophages in the heart, without affecting other types of immune cells. In cardiac fibroblasts that underwent cyclic stretching, empagliflozin pre-treatment diminished the expression of C-C motif chemokine ligand 2 (CCL2), the ligand for CCR2.
Empagliflozin’s ability to suppress CCL2 expression in fibroblasts and curtail the recruitment of CCR2+ macrophages may represent a potential strategy to decelerate heart failure progression. These findings advance our understanding of the cardioprotective mechanisms of SGLT2 inhibitors and propose novel therapeutic avenues for heart failure management.
More abstracts on this topic:
ANSWER-HF: A Randomized Controlled Trial of Sacubitril/Valsartan Versus Enalapril in Patients with Chronic Chagas Cardiomyopathy and Reduced Ejection Fraction
Madrini Junior Vagner, Antunes Talita, Damiani Lucas, Jose Grupi Cesar, Mathias Junior Wilson, Lopes Renato, Ramires Felix, Ramos Souza Paulo, Fernandes Fabio, Maria Ianni Barbara, Silva Martins Alan, JadÁn Luzuriaga Georgina Del Cisne, Cardoso Barbosa Fonseca Keila, Ribeiro Orlando, Bispo Da Cruz Allecineia
A Machine Learning Algorithm to Detect Pediatric Supraventricular Tachycardia Risk from Baseline ECGsArezoumand Amirhossein, Danala Gopichandh, Masnadi Khiabani Parisa, Ebert David, Behere Shashank