Final ID: Mo3018
Safety of Dofetilide Initiation Following a Shortened Amiodarone Washout Period
Abstract Body (Do not enter title and authors here): Background: Dofetilide, a commonly prescribed class III antiarrhythmic for atrial fibrillation (AF), currently requires a 3-month amiodarone washout period per FDA guidelines. We evaluated the safety of initiating dofetilide within this 3-month washout period.
Methods: We performed a retrospective cohort study across all Mayo Clinic sites and identified patients using centralized datamart that were transitioned from amiodarone to dofetilide in less than 90 days. Patients were included and confirmed by manual chart review if they had been on amiodarone for ≥ 12 weeks and if available amiodarone level >0.3 mg/ml at the time of dofetilide initiation. Serial ECGs were assessed during the hospitalization for dofetilide initiation and at 3-, 6-, and 12-months follow-up to evaluate QT interval. Primary safety end point was torsade de pointes.
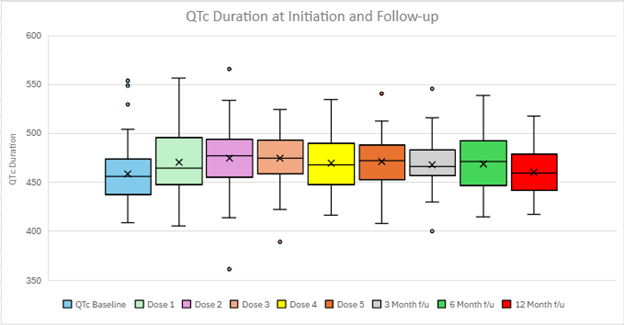
Results: The final cohort included 66 patients with a mean age of 65 ±14 years; 71.0% were male; and the average BMI was 29.6 ±5.1 kg/m2. Of the 66 patients, 65 had a history of AF: 31.8% paroxysmal, 54.5% persistent, and 12.1% long-standing persistent. Permanent pacemakers and ICDs were present in 27.3% and 22.7% of patients. Patients received amiodarone for a median of 7.8 months at a mean daily dose of 187.1±48.3 mg (range: 100 – 400 mg), with a mean transition period of 44.2±20.3 days. Of 66 patients, 16 (24 %) transitioned within 30 days of stopping amiodarone—including 5 (8 %) within 14 days—while the remaining 50 (76 %) was between 30 and 90 days. A total of 24.2% (16/66) patients required dofetilide dose reduction due to QT prolongation and 10.6% of patients (7/66) required drug discontinuation. There was no difference in either discontinuation rate or dose reduction rate in patients with a transition period ≤30 days vs > 30 days [6.25% vs. 12% (p=0.85) and 12.5% vs. 28% (p=0.36), respectively]. No cases of torsades de pointes were observed during dofetilide initiation or follow up.
Conclusion: In this cohort, dofetilide initiation less than 90 days after amiodarone discontinuation was not associated with increased torsades de pointes risk. These data challenge and support reconsideration of the blanket 3-month washout and suggest that a monitored, expedited rhythm-control transition is feasible and safe.
Methods: We performed a retrospective cohort study across all Mayo Clinic sites and identified patients using centralized datamart that were transitioned from amiodarone to dofetilide in less than 90 days. Patients were included and confirmed by manual chart review if they had been on amiodarone for ≥ 12 weeks and if available amiodarone level >0.3 mg/ml at the time of dofetilide initiation. Serial ECGs were assessed during the hospitalization for dofetilide initiation and at 3-, 6-, and 12-months follow-up to evaluate QT interval. Primary safety end point was torsade de pointes.
Results: The final cohort included 66 patients with a mean age of 65 ±14 years; 71.0% were male; and the average BMI was 29.6 ±5.1 kg/m2. Of the 66 patients, 65 had a history of AF: 31.8% paroxysmal, 54.5% persistent, and 12.1% long-standing persistent. Permanent pacemakers and ICDs were present in 27.3% and 22.7% of patients. Patients received amiodarone for a median of 7.8 months at a mean daily dose of 187.1±48.3 mg (range: 100 – 400 mg), with a mean transition period of 44.2±20.3 days. Of 66 patients, 16 (24 %) transitioned within 30 days of stopping amiodarone—including 5 (8 %) within 14 days—while the remaining 50 (76 %) was between 30 and 90 days. A total of 24.2% (16/66) patients required dofetilide dose reduction due to QT prolongation and 10.6% of patients (7/66) required drug discontinuation. There was no difference in either discontinuation rate or dose reduction rate in patients with a transition period ≤30 days vs > 30 days [6.25% vs. 12% (p=0.85) and 12.5% vs. 28% (p=0.36), respectively]. No cases of torsades de pointes were observed during dofetilide initiation or follow up.
Conclusion: In this cohort, dofetilide initiation less than 90 days after amiodarone discontinuation was not associated with increased torsades de pointes risk. These data challenge and support reconsideration of the blanket 3-month washout and suggest that a monitored, expedited rhythm-control transition is feasible and safe.
More abstracts on this topic:
Quality of Amiodarone Monitoring in a Veterans Affairs Healthcare System
Wang Anna, Ahn Joy, Morimoto Cherie, Yuge Dora, Han Janet, Jackevicius Cynthia
ECG correlates of chronic right ventricular pacing: QT interval predicts pacing-induced cardiomyopathy while QRS duration predicts cardiac resynchronization therapy responseGupta Amulya, Shahab Ahmed, Harvey Christopher, Sheldon Seth, Reddy Madhu, Noheria Amit