Final ID: MP2510
Race does not influence the efficacy and safety of mineralocorticoid-receptor antagonists in heart failure: An individual-participant data meta-analysis of 4 trials
OBJECTIVES: We examined the efficacy and safety of MRAs, compared with placebo, in patients with HF and reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF), according to race (Black or non-Black).
METHODS: We conducted an individual-participant data meta-analysis of the 4 major randomized controlled trials comparing MRAs to placebo in patients with HFrEF (RALES, EMPHASIS-HF) and HFpEF (TOPCAT, FINEARTS-HF). Race was self-reported. The primary outcome was a composite of cardiovascular death or first HF hospitalization.
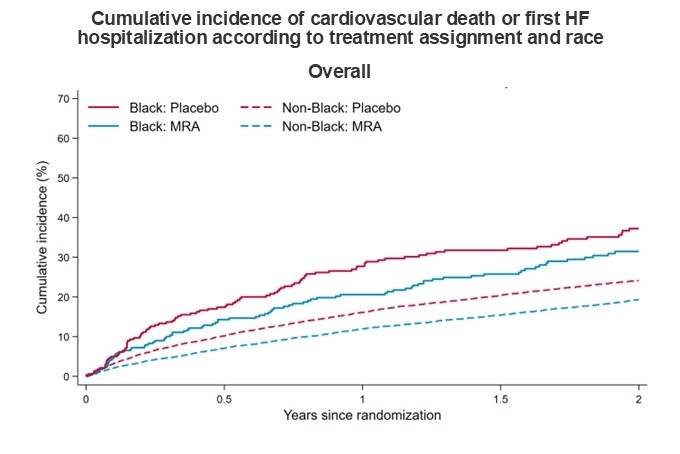
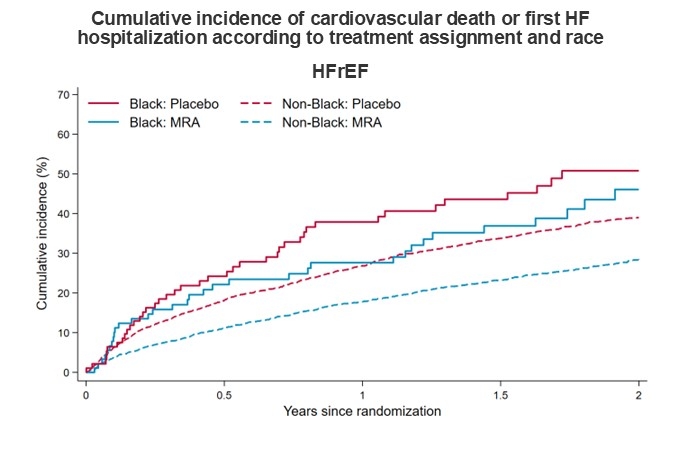
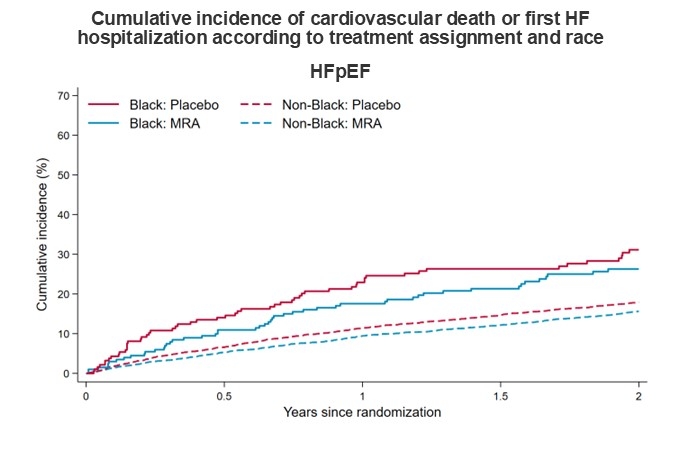
RESULTS: Of the 13,846 patients randomized in the four trials, 577 (4.2%) identified as Black (4.3% in the HFrEF trials; 4.1% in the HFpEF trials). Rates of HF hospitalizations and death were higher in Black than non-Black patients. The hazard ratio (HR) for MRA versus placebo for the primary composite outcome was 0.87 (95% CI, 0.66-1.15) in Black patients and 0.77 (95% CI, 0.72-0.82) in non-Black patients (Pinteraction=0.34) (Figure). For first HF hospitalization, the HRs were 0.86 (95% CI, 0.63-1.17) and 0.73 (95% CI, 0.68-0.80) for Black and non-Black patients, respectively (Pinteraction=0.36). The corresponding HRs for cardiovascular death were 0.75 (95% CI, 0.48-1.17) and 0.81 (95% CI, 0.74-0.90) respectively (Pinteraction=0.80). For cardiovascular death and total HF hospitalizations, the corresponding rate ratios were 0.80 (0.61-1.06) and 0.76 (95% CI, 0.71-0.82), respectively (Pinteraction=0.96). Adverse events with MRAs, compared with placebo, including hypotension, elevated creatinine, hyperkalemia, and hypokalemia were not modified by race. Findings were similar when the population was restricted to patients randomized in the Americas only. The effects of MRAs in patients with HFrEF and HFpEF, individually, were not modified by race (Figure).
CONCLUSIONS: The beneficial effects of MRAs, compared with placebo, on clinical events were comparable in Black and non-Black patients with HF, regardless of HF phenotype.
- Butt, Jawad ( Rigshospitalet , Copenhagen , Denmark )
- Pitt, Bertram ( University of Michigan School , Ann Arbor , Michigan , United States )
- Senni, Michele ( ASST PAPA GIOVANNI XXIII , Bergamo , Italy )
- Shah, Sanjiv ( NORTHWESTERN UNIVERSITY , Chicago , Illinois , United States )
- Zannad, Faiez ( INSERM-CIC , Vandoeuvre Les Nancy , France )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Jhund, Pardeep ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Henderson, Alasdair David ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Talebi, Atefeh ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Vardeny, Orly ( Minneapolis VA Health Care System , Minneapolis , Minnesota , United States )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Desai, Akshay ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Lam, Carolyn ( NATIONAL HEART CENTRE SINGAPORE , Singapore , Singapore )
Meeting Info:
Session Info:
(Non-) Roid Rage: Novel Insights Into Non-Steroidal MRAs for HF
Monday, 11/10/2025 , 12:15PM - 01:25PM
Moderated Digital Poster Session
More abstracts on this topic:
Kondo Toru, Amarante Flaviana, Lam Carolyn, Senni Michele, Shah Sanjiv, Voors Adriaan, Zannad Faiez, Pitt Bertram, Vaduganathan Muthiah, Solomon Scott, Mcmurray John, Jhund Pardeep, Henderson Alasdair David, Claggett Brian, Desai Akshay, Brinker Meike, Lay-flurrie James, Schloemer Patrick, Viswanathan Prabhakar
Eplerenone treatment limit electrocardiogram alteration in a non-diabetic chronic kidney disease rat model.Soulie Matthieu, Sanchez-bayuela Tania, Cambier Agathe, Jaisser Frederic
More abstracts from these authors:
Vardeny Orly, Zannad Faiez, Pitt Bertram, Lay-flurrie James, Viswanathan Prabhakar, Horvat-broecker Andrea, Scalise Andrea, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Claggett Brian, Desai Akshay, Jhund Pardeep, Lam Carolyn, Senni Michele, Shah Sanjiv, Voors Adriaan
Efficacy of finerenone in patients with heart failure and mildly reduced or preserved ejection fraction: A prespecified analysis of heart rate in the FINEARTS-HF trialChimura Misato, Senni Michele, Zannad Faiez, Pitt Bertram, Vaduganathan Muthiah, Solomon Scott, Mcmurray John, Jhund Pardeep, Henderson Alasdair David, Claggett Brian, Desai Akshay, Lay-flurrie James, Scalise Andrea, Rohwedder Katja, Lam Carolyn