Final ID: MP2211
Tirzepatide Reduces the Risk of Major Adverse Cardiovascular Events: An Individual Participant Data Meta-analysis of Phase 3 Randomized Clinical Trials
Abstract Body (Do not enter title and authors here):
Background: Individual participant data (IPD) from Phase 3 trials of tirzepatide offer a unique opportunity to evaluate its CV efficacy and identify response correlates. These insights may guide the clinical use of the drug while we await results from CVOTs, particularly in examining its CV effectiveness.
Aim: In this IPD meta-analysis, we sought to assess the CV efficacy of tirzepatide and identify predictors of treatment response.
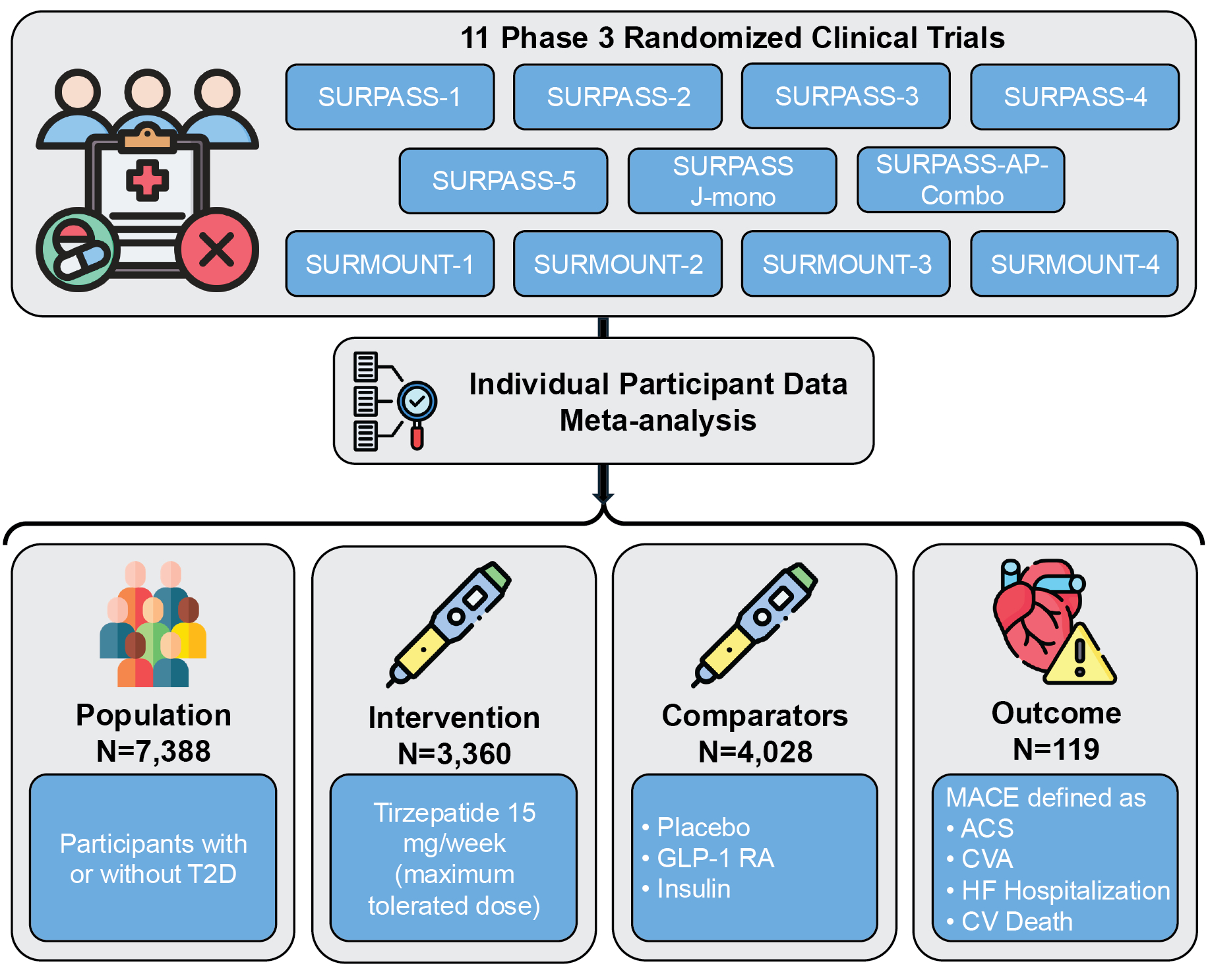
Methods: We pooled individual-level data from 11 Phase 3 RCTs comparing tirzepatide with placebo, insulin, or GLP-1 RAs in participants with or without T2D to assess tirzepatide’s CV efficacy. In the intervention arm, participants who received tirzepatide 15 mg/week—the maximum tolerated dose—were included and compared against pooled comparators, in line with ongoing CVOTs. MACE was a composite of adjudicated ACS, CVA, HF hospitalization, and CV death. We used a mixed-effects Cox proportional hazards model to model MACE, with the intervention as a fixed effect and the trial as a random effect. Inverse probability weighting (IPW) was employed to account for study design differences across RCTs. To identify response correlates within the tirzepatide arm, we analyzed the hazard of MACE for those who showed improvement in cardiometabolic components vs. those who did not.
Results: There were 3,360 individuals in the tirzepatide 15 mg/week and 4,028 in the comparator arms across trials, with a median age of 55 years [IQR, 46-63] and 52% women. Over a median follow-up of 56 weeks [44-76], 119 (1.6%) experienced MACE. Tirzepatide 15 mg/week significantly reduced the risk of MACE (HR, 0.67 [95% CI, 0.51-0.87]), with an IPW-weighted incidence of MACE of 1.1% in the tirzepatide and 1.7 % in the comparator arms. Tirzepatide’s CV efficacy was consistent across subgroups, with more pronounced MACE benefits among individuals with vs. without obesity (P<0.001). In the tirzepatide arm, the hazard of MACE was up to 81% lower in participants who experienced improvements in weight, waist circumference (WC), diastolic blood pressure (DBP), and fasting blood glucose (FBG), among other cardiometabolic components, compared with those without such improvements.
Conclusion: Tirzepatide 15 mg/week reduces the risk of MACE by 33% with consistent efficacy across subgroups. Weight loss and reductions in WC, DBP, and FBG during tirzepatide therapy suggest potential long-term benefits for MACE, highlighting their role in personalized treatment recommendations.
Background: Individual participant data (IPD) from Phase 3 trials of tirzepatide offer a unique opportunity to evaluate its CV efficacy and identify response correlates. These insights may guide the clinical use of the drug while we await results from CVOTs, particularly in examining its CV effectiveness.
Aim: In this IPD meta-analysis, we sought to assess the CV efficacy of tirzepatide and identify predictors of treatment response.
Methods: We pooled individual-level data from 11 Phase 3 RCTs comparing tirzepatide with placebo, insulin, or GLP-1 RAs in participants with or without T2D to assess tirzepatide’s CV efficacy. In the intervention arm, participants who received tirzepatide 15 mg/week—the maximum tolerated dose—were included and compared against pooled comparators, in line with ongoing CVOTs. MACE was a composite of adjudicated ACS, CVA, HF hospitalization, and CV death. We used a mixed-effects Cox proportional hazards model to model MACE, with the intervention as a fixed effect and the trial as a random effect. Inverse probability weighting (IPW) was employed to account for study design differences across RCTs. To identify response correlates within the tirzepatide arm, we analyzed the hazard of MACE for those who showed improvement in cardiometabolic components vs. those who did not.
Results: There were 3,360 individuals in the tirzepatide 15 mg/week and 4,028 in the comparator arms across trials, with a median age of 55 years [IQR, 46-63] and 52% women. Over a median follow-up of 56 weeks [44-76], 119 (1.6%) experienced MACE. Tirzepatide 15 mg/week significantly reduced the risk of MACE (HR, 0.67 [95% CI, 0.51-0.87]), with an IPW-weighted incidence of MACE of 1.1% in the tirzepatide and 1.7 % in the comparator arms. Tirzepatide’s CV efficacy was consistent across subgroups, with more pronounced MACE benefits among individuals with vs. without obesity (P<0.001). In the tirzepatide arm, the hazard of MACE was up to 81% lower in participants who experienced improvements in weight, waist circumference (WC), diastolic blood pressure (DBP), and fasting blood glucose (FBG), among other cardiometabolic components, compared with those without such improvements.
Conclusion: Tirzepatide 15 mg/week reduces the risk of MACE by 33% with consistent efficacy across subgroups. Weight loss and reductions in WC, DBP, and FBG during tirzepatide therapy suggest potential long-term benefits for MACE, highlighting their role in personalized treatment recommendations.
More abstracts on this topic:
A Randomized Phase 2 Trial of Muvalaplin: An Oral Disrupter of the Assembly of Lipoprotein(a) Particles
Nicholls Stephen, Ni Wei, Rhodes Grace, Nissen Steven, Navar Ann Marie, Michael Laura, Krege John
Association between accelerometer-corrected physical activity and the prevalence of hypertension, diabetes, and obesity: Results from Korea National Health and Nutrition Examination Survey, 2014-2017Shin Woo Young, Choi Ji-yeob, Lee Hyo, Lee Miyoung