Final ID: 4361027
Bempedoic acid monotherapy, LDL cholesterol and cardiovascular events: a secondary analysis of the CLEAR Outcomes trial
Research Questions: Does bempedoic acid monotherapy decrease MACE compared with placebo? What is the LDL-C lowering effect of bempedoic acid monotherapy at 6 months?
Methods: CLEAR Outcomes randomized 13970 statin-intolerant adults at high risk for, or with established CV disease to bempedoic acid 180 mg or placebo. Fifty-nine percent of participants (8217) were not taking any LLT at baseline. In this subpopulation, we assessed the impact of bempedoic acid on the primary composite outcome of nonfatal myocardial infarction (MI), coronary revascularization, nonfatal stroke, CV death (MACE4) and key secondary outcomes including the composite of nonfatal MI, nonfatal stroke, CV death (MACE3), the individual components of MACE4, and all-cause death in a time-to-event manner using cox proportional hazards. Additionally, we assessed LDL-C change after 6 months of therapy.
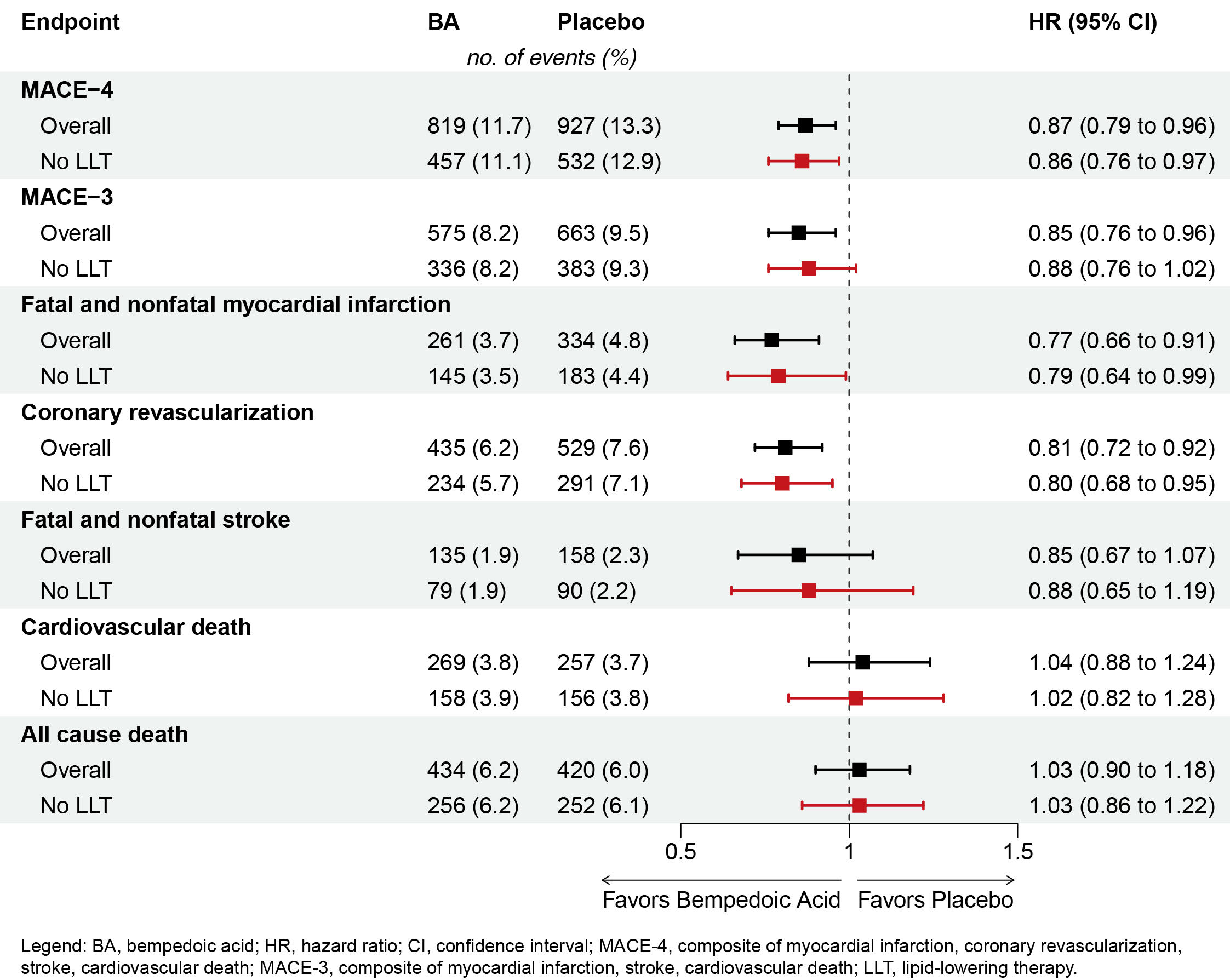
Results: Compared with placebo, bempedoic acid monotherapy reduced MACE4 by 14% (HR 0.86; 95% CI, 0.76 – 0.97). Other key secondary outcomes demonstrated similar efficacy of bempedoic acid monotherapy compared with the overall trial population (Figure). Bempedoic acid monotherapy lowered LDL-C by -20.6% (95% CI, -21.7 to -19.6%) compared with placebo at 6 months. Serious adverse events (AE) were reported in 27.9% and 27.1% of patients who received bempedoic acid monotherapy and placebo, respectively. AEs that led to study drug discontinuation occurred in 11.3% and 11.0% of patients, respectively.
Conclusions: Bempedoic acid demonstrates similar efficacy and safety when taken as monotherapy compared with all participants in CLEAR Outcomes. These data support its use as monotherapy to reduce CV events in statin-intolerant patients with high CV risk.
More abstracts on this topic:
Wilsgaard Tom, Rosamond Wayne, Schirmer Henrik, Lindekleiv Haakon, Attia Zachi, Lopez-jimenez Francisco, Leon David, Iakunchykova Olena
A transformative LDL cholesterol–lowering in vivo CRISPR gene editing medicine that functionally upregulates LDLR in mice and non-human primatesNewmark Judith, Raghav Jimit, Jaskolka Michael, Diner Benjamin, Soman Vikram, Jinadasa Tushare, Apte Ameya, Wu Meng, Bottega Steve, Thakkar Mansi, Agosto Luis, Wrighton Paul, Majithia Deep, Jambard Shreya, Jansson-fritzberg Linnea, Jones Mark, Fletcher Jillian, Weiss Mckenzie, Kaye Emily, Steward Briana, Bochicchio James, Pietrasiewicz Stephen, Iovino Salvatore, Marco Rubio Eugenio, Trong Phan Huu, Chander Nisha, Kazemian Mohammadreza, Lam Kieu, Reid Steve, Dinsmore Michael, Teslovich Tanya, Xie Jenny, Gupta Anshul, Amin Parth, Burkly Linda, Thompson Morgan, Rizal Salu, Bilodeau Maxime, Dong Ruhong, Zhen Wei