Final ID: 4360915
Association Between Direct Oral Anticoagulant Dose and Risk of Stroke/Systemic Embolism, Major Bleeding, or All-Cause Mortality in Patients with Ischemic Stroke and Atrial Fibrillation
Methods: We included ischemic stroke patients admitted to a GWTG-Stroke hospitals in Oct. 2012-Dec. 2019 who were >66 years, had a history of AF/flutter, and were discharged on a DOAC (dabigatran, rivaroxaban, or apixaban). DOAC dosing was defined as appropriate (standard dose or appropriately adjusted dose), underdose (reduced dose without indications for dose reduction or dose lower than recommended), or overdose (standard dose in patients with indications for dose reduction or dose higher than recommended). Normalized inverse probability weighted generalized boosted models were used to estimate 1-year outcomes after discharge.
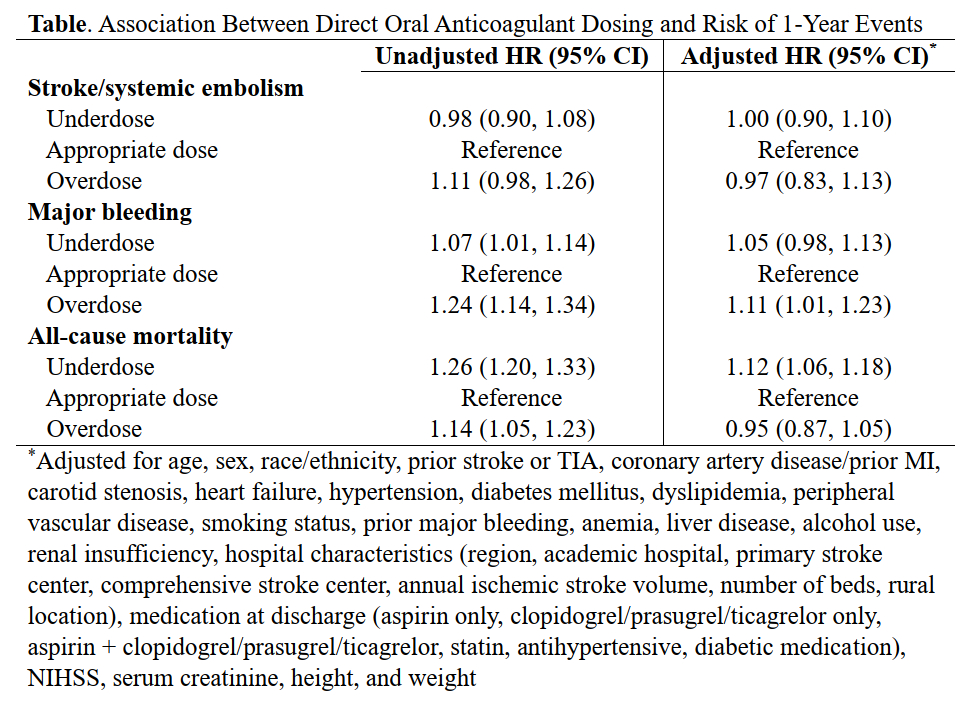
Results: Of the 37,464 ischemic stroke survivors (median age: 81 years; 53% female; 7% non-Hispanic Black), 68% were discharged with the appropriate DOAC dose, 22% were underdosed and 10% were overdosed. Apixaban was the most common DOAC (68%), followed by rivaroxaban (23%) and dabigatran (9%). The cumulative 1-year incidence of stroke/systemic embolism were 7.1%, 7.0%, and 7.9% among patients who were appropriate dosed, underdosed, and overdosed. After multivariable adjustments, there was no significant association between DOAC dose and stroke/systemic embolism (Table). However, patients who were overdosed (aHR 1.11 [1.01-1.23]) were associated with greater risk of major bleeding compared with appropriately dosed patients. Furthermore, patients who were underdosed were associated with greater risk of all-cause mortality (aHR 1.12 [1.06-1.18]). No significant association with mortality risk was observed in those who were overdosed.
Conclusion: In a nationwide registry of ischemic stroke survivors with AF, approximately one-third of patients were prescribed a non-recommended DOAC dose. Patients who were overdosed had greater risk of major bleeding, while those underdosed had a greater risk of all-cause mortality. Strategies, including medical education, to ensure appropriate DOAC dosing at discharge are needed to reduce risk of adverse outcomes.
- Wang, Wendy ( UT Southwestern Medical Center , Dallas , Texas , United States )
- Schwamm, Lee ( Yale School of Medicine , New Haven , Connecticut , United States )
- Maisch, Lesley ( Patient-Centered Research Into Outcomes Stroke Patients Prefer and Effectiveness Research Study , Durham , North Carolina , United States )
- Hannah, Deidre ( Patient-Centered Research Into Outcomes Stroke Patients Prefer and Effectiveness Research Study , Durham , North Carolina , United States )
- Lopes, Renato ( DUKE CLINICAL RESEARCH , Durham , North Carolina , United States )
- Xian, Ying ( UTSW , Dallas , Texas , United States )
- Ayodele, Iyanuoluwa ( DCRI , Jonesboro , Georgia , United States )
- Laskowitz, Daniel ( Duke University , Durham , North Carolina , United States )
- Obrien, Emily ( Duke University , Durham , North Carolina , United States )
- Peterson, Eric ( UT Southwestern Medical Center , Dallas , Texas , United States )
- Matsouaka, Roland ( Duke University , Durham , North Carolina , United States )
- Smith, Eric ( UNIVERSITY OF CALGARY , Calgary , Alberta , Canada )
- Bhatt, Deepak ( Mount Sinai Fuster Heart Hospital , Scarsdale , New York , United States )
- Fonarow, Gregg ( UCLA MEDICAL CENTER , Los Angeles , California , United States )
Meeting Info:
Session Info:
Brain, Heart, and Risk: Advancing Cognitive and Cardiovascular Protection
Saturday, 11/08/2025 , 01:30PM - 02:35PM
Abstract Oral Session
More abstracts on this topic:
Wong Ka-ho, Krothapalli Neeharika, Littig Lauren, Champagne Alison, Majersik Jennifer, Reddy Vivek, De Havenon Adam
A Real-world Evaluation of Longitudinal Healthcare Expenses in a Health System Registry of Type-2 Diabetes Mellitus and Cardiovascular Disease Enabled by the 21st Century Cures ActDhingra Lovedeep, Aminorroaya Arya, Pedroso Aline, Rajpura Jigar, Mehanna Sherif, Tonnu-mihara Ivy, Khera Rohan
More abstracts from these authors:
Wang Duanduan, Smith Eric, Bhatt Deepak, Ayodele Iyanuoluwa, Matsouaka Roland, Obrien Emily, Xian Ying, Sherrod Charles, Chan Paul, Laskowitz Daniel, Peterson Eric, Fonarow Gregg, Schwamm Lee
Antithrombotic Therapy for Secondary Stroke Prevention in Patients with Severe Chronic Kidney Disease and Atrial FibrillationJones Erica, Peterson Eric, Xian Ying, Ayodele Iyanuoluwa, Obrien Emily, Laskowitz Daniel, Fonarow Gregg, Matsouaka Roland, Schwamm Lee, Smith Eric, Bhatt Deepak