Final ID: MP1893
Intravenous Administration of Muse Cells Promotes Long-Lasting Functional Recovery in a Rat Model of Spinal Cord Ischemic Injury
Abstract Body (Do not enter title and authors here): Introduction Spinal cord ischemic injury (SCII) is a devastating complication of aortic surgery that often leads to irreversible motor dysfunction. Despite its clinical severity, no effective treatment has been established. Multilineage-differentiating stress-enduring (Muse) cells are a non-tumorigenic, endogenous pluripotent-like stem cell population marked by SSEA-3 expression, with emerging evidence supporting their regenerative potential. However, their long-term therapeutic efficacy in SCII remains largely unexplored.
Hypothesis We hypothesized that intravenous administration of Muse cells would promote sustained motor function recovery in a rat model of severe SCII.
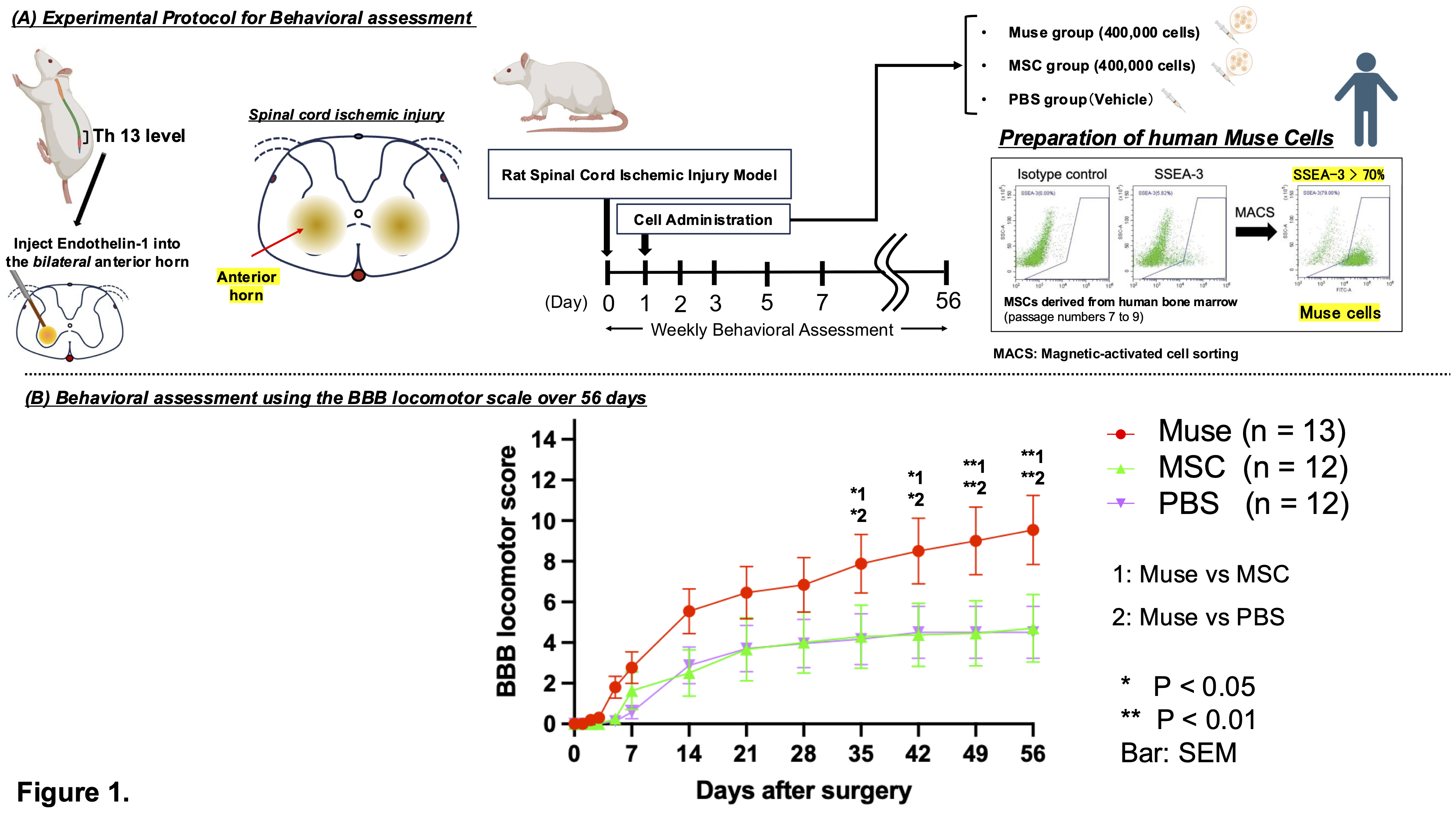
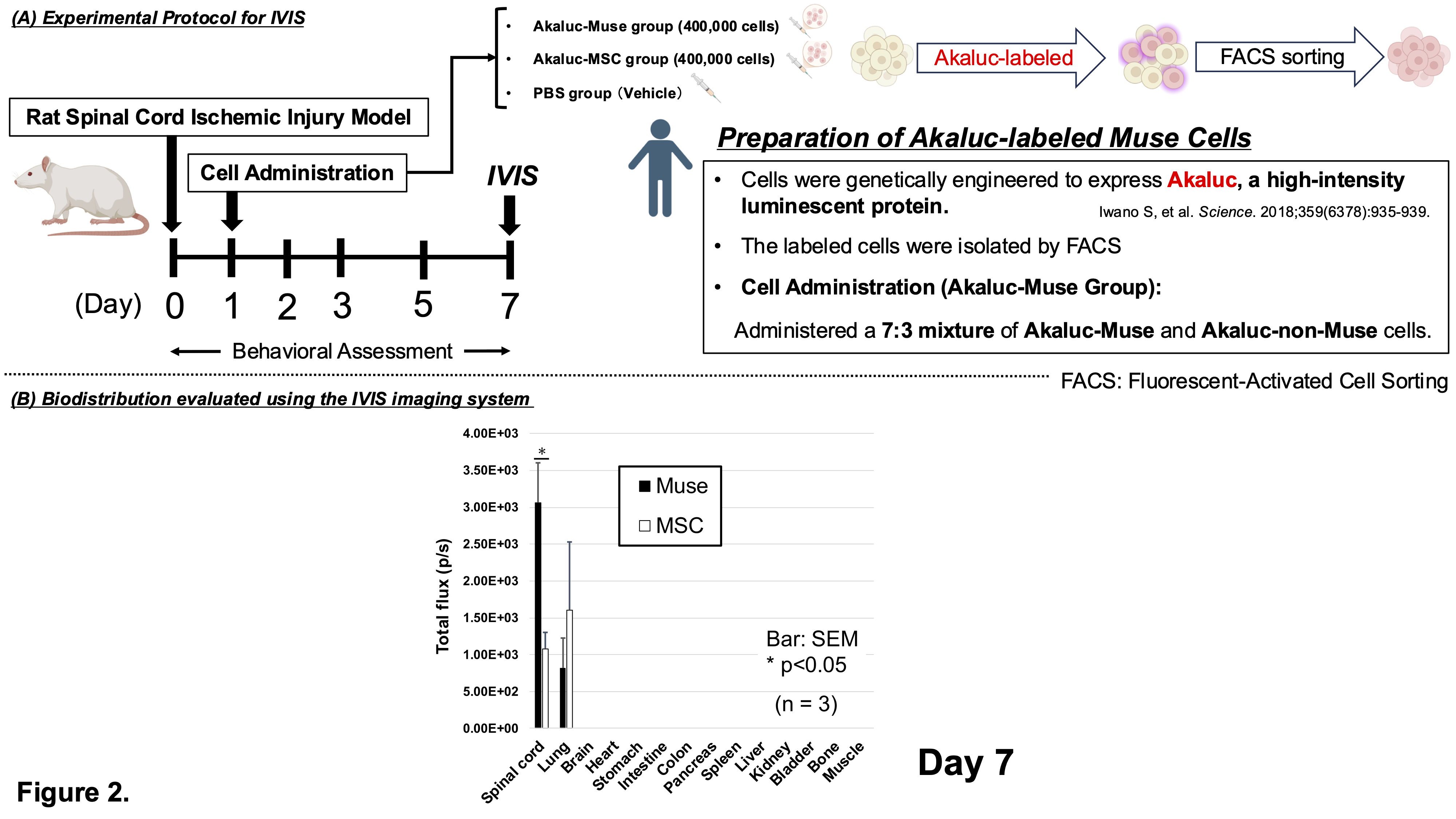
Methods Severe SCII was induced in eight-week-old male Wistar rats by bilaterally injecting endothelin-1 into the anterior horns of the 13th thoracic spinal cord segment. Muse cells were isolated from human bone marrow-derived mesenchymal stem cells (MSCs). At 24 hours post-injury, rats with a BBB locomotor score of 0 were randomly assigned to receive Muse cells, MSCs, or PBS via penile vein injection. Motor function was evaluated for 8 weeks. No immunosuppressants were administered. IVIS imaging and immunofluorescence were performed to assess in vivo biodistribution and engraftment. Cell differentiation was evaluated by double immunostaining with human-specific and lineage-specific markers.
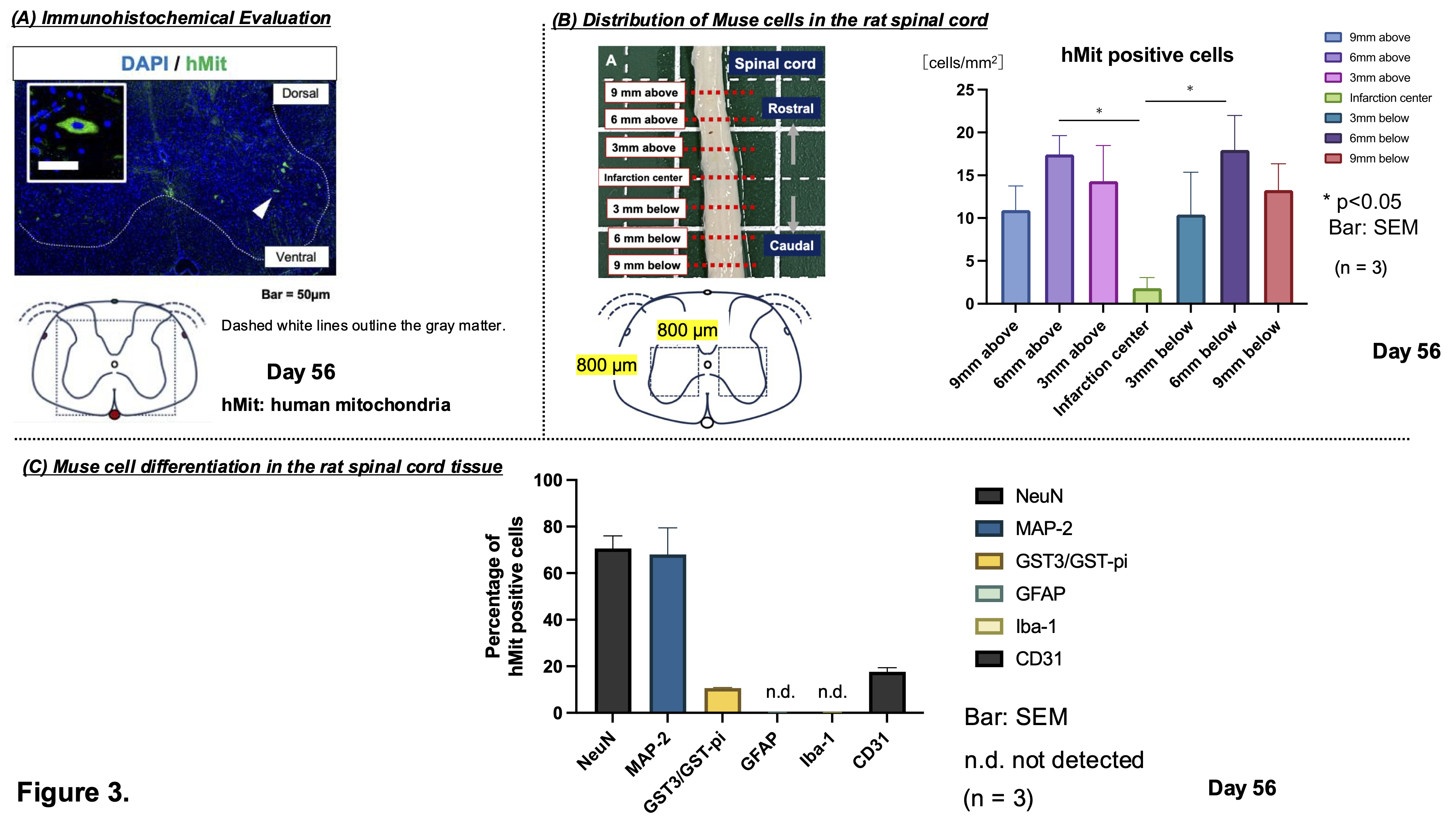
Results At 8 weeks post-treatment, the Muse group showed significantly improved motor recovery compared to MSC and PBS groups (BBB score: Muse 9.5±1.7; MSC 4.7±1.7; PBS 4.5±1.3; p<0.01). IVIS imaging at 1 week revealed stronger luminescence signals in the spinal cords of Muse-treated rats (p<0.05), suggesting selective migration to the injury site. Human mitochondria-positive cells were mainly located 6 mm rostral and caudal to the lesion center (T13), indicating peri-lesional accumulation (p<0.05). Double immunostaining confirmed differentiation into neurons (NeuN: 70.6±5.4%), neuronal dendrites (MAP-2: 68.0±11.4%), oligodendrocytes (GST-pi: 10.6±0.3%), and vascular endothelial cells (CD31: 17.6±1.8%).
Conclusions Intravenous administration of human Muse cells significantly enhanced and maintained hindlimb motor recovery in a rat model of SCII without immunosuppression. Muse cells homed to the damaged spinal cord and differentiated into neural and vascular lineages, contributing to structural and functional repair. These findings highlight Muse cells as a promising therapeutic approach for acute SCII.
Hypothesis We hypothesized that intravenous administration of Muse cells would promote sustained motor function recovery in a rat model of severe SCII.
Methods Severe SCII was induced in eight-week-old male Wistar rats by bilaterally injecting endothelin-1 into the anterior horns of the 13th thoracic spinal cord segment. Muse cells were isolated from human bone marrow-derived mesenchymal stem cells (MSCs). At 24 hours post-injury, rats with a BBB locomotor score of 0 were randomly assigned to receive Muse cells, MSCs, or PBS via penile vein injection. Motor function was evaluated for 8 weeks. No immunosuppressants were administered. IVIS imaging and immunofluorescence were performed to assess in vivo biodistribution and engraftment. Cell differentiation was evaluated by double immunostaining with human-specific and lineage-specific markers.
Results At 8 weeks post-treatment, the Muse group showed significantly improved motor recovery compared to MSC and PBS groups (BBB score: Muse 9.5±1.7; MSC 4.7±1.7; PBS 4.5±1.3; p<0.01). IVIS imaging at 1 week revealed stronger luminescence signals in the spinal cords of Muse-treated rats (p<0.05), suggesting selective migration to the injury site. Human mitochondria-positive cells were mainly located 6 mm rostral and caudal to the lesion center (T13), indicating peri-lesional accumulation (p<0.05). Double immunostaining confirmed differentiation into neurons (NeuN: 70.6±5.4%), neuronal dendrites (MAP-2: 68.0±11.4%), oligodendrocytes (GST-pi: 10.6±0.3%), and vascular endothelial cells (CD31: 17.6±1.8%).
Conclusions Intravenous administration of human Muse cells significantly enhanced and maintained hindlimb motor recovery in a rat model of SCII without immunosuppression. Muse cells homed to the damaged spinal cord and differentiated into neural and vascular lineages, contributing to structural and functional repair. These findings highlight Muse cells as a promising therapeutic approach for acute SCII.
More abstracts on this topic:
Dual Pathway Inhibition prescription after percutaneous vascular intervention is low, and dependent on physician and treating facility practice patterns
Jarosinski Marissa, Lowenkamp Mikayla, Madigan Michael, Reitz Katherine, Chaer Rabih, Sridharan Natalie
Apolipoprotein A2 as Protection Against Increased Mortality After Aortic Aneurysm RepairEguchi Miu, Nakamura Takamitsu, Omori Kazuhira, Horikoshi Takeo, Yoshizaki Toru, Kobayashi Tsuyoshi, Sato Akira