Final ID: MP2779
Mavacamten monotherapy in real-world patients with obstructive hypertrophic cardiomyopathy: Evidence from COLLIGO-HCM
Purpose: To describe the real-world outcomes of patients treated with mavacamten monotherapy.
Methods: This retrospective study uses patient-level data from existing medical records and electronic registries from COLLIGO-HCM sites in the US, Canada, the UK, Australia, and Israel. Patient characteristics, NYHA class, echocardiography data, and safety were analyzed at baseline and follow-up visits, up to week 36 in four cohorts: monotherapy group (n=88), defined as those with monotherapy at initiation (n=20) plus monotherapy after discontinuation of background therapy (n=68), mavacamten in combination with background therapy that has been reduced (n=13), mavacamten in combination with stable, unchanged background therapy over the duration of the study period (n=177), and the overall cohort (n= 278).
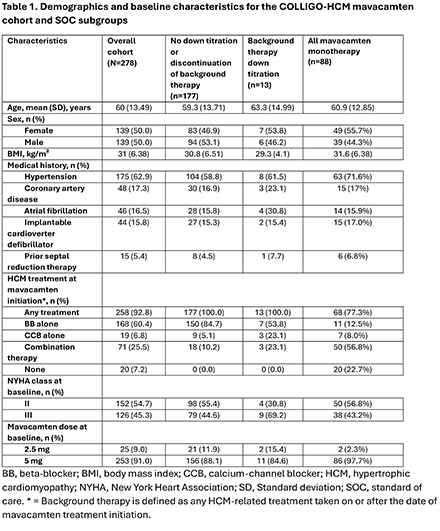
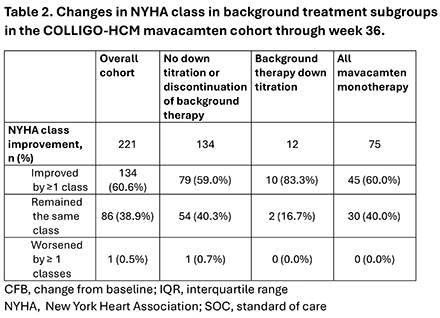
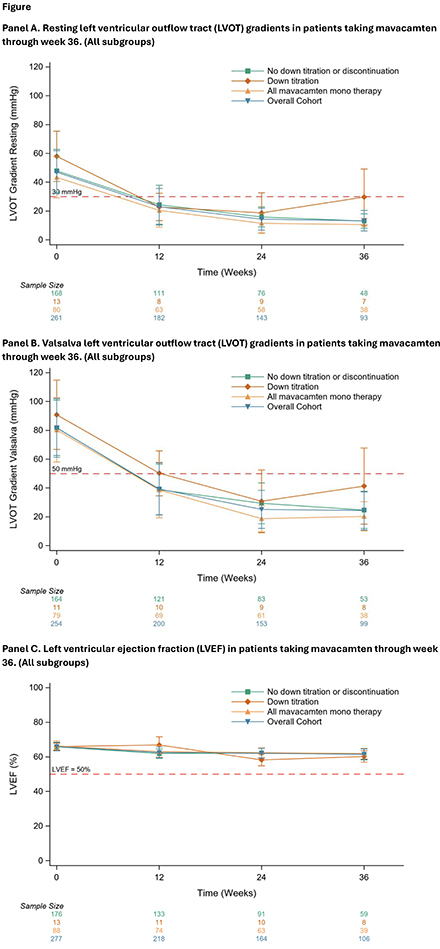
Results: The mavacamten monotherapy cohort included 88/278 patients (31.7%). Baseline clinical and demographic characteristics include: 55.7% female, 56.8% NYHA Class II and 43.2% Class III, high BMI (mean: 31.6 kg/m2), history of atrial fibrillation (15.9%) and hypertension (71.6%). The median follow-up time was 35.9 weeks (IQR:21.9,116.9) (Table 1). By week 36, 60% of patients achieved ≥ 1 NYHA class improvement. Additionally, mean left ventricular outflow tract (LVOT) gradients of ≤30 mm Hg at rest and with Valsalva were achieved in 97.3% and 81.6% of patients, respectively (Table 2. Figure). Mean left ventricular ejection fraction (LVEF) post mavacamten initiation remained at or above 62% throughout follow-up (baseline value 66.4%). Two patients in the monotherapy cohort permanently discontinued mavacamten due to LVEF<50%, both recovered after discontinuing treatment.
Conclusions: This analysis demonstrates effectiveness, safety and consistency of mavacamten monotherapy with previously reported monotherapy analyses from pivotal, long-term and real-world studies.
- Bilen, Ozlem ( Emory University , Atlanta , Georgia , United States )
- Pruett, Cliff ( University of Texas Southwestern Medical Center , Dallas , Texas , United States )
- Burford, Edward ( Guy's and St Thomas' NHS Foundation Trust , London , United Kingdom )
- Maksabedian Hernandez, Ervant ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Han, Eileen ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Schuler, Patricia ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Sandler, Belinda ( Bristol-Myers Squibb , Uxbridge , United Kingdom )
- Li, Leanne ( IQVIA , Durham , North Carolina , United States )
- Banks, Victoria ( IQVIA , London , United Kingdom )
- Arora, Pankaj ( University of Alabama , Birmingham , Alabama , United States )
- Adler, Arnon ( Toronto General Hospital , Toronto , Ontario , Canada )
- Bastiaenen, Rachel ( Guy's and St Thomas' NHS Foundation Trust , London , United Kingdom )
- Macnamara, James ( University of Texas Southwestern Medical Center , Dallas , Texas , United States )
- Paratz, Elizabeth ( St Vincent’s Hospital & St Vincent's Private Hospital , Melbourne , Victoria , Australia )
- Maor, Elad ( Sheba Medical Center , Ramat Gan , Tel HaShomer , Israel )
- Arad, Michael ( Sheba Medical Center , Ramat Gan , Tel HaShomer , Israel )
- Gold, Matthew ( Emory University , Atlanta , Georgia , United States )
- Patel, Nirav ( University of Alabama , Birmingham , Alabama , United States )
Meeting Info:
Session Info:
Innovation & Precision Medicine in Hypertrophic Cardiomyopathy
Monday, 11/10/2025 , 10:45AM - 11:35AM
Moderated Digital Poster Session
More abstracts on this topic:
Sangha Veer, Aminorroaya Arya, Dhingra Lovedeep, Pedroso Aline, Oikonomou Evangelos, Khera Rohan
Acacetin's Antiarrhythmic Potential in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Harboring Hypertrophic and Dilated Cardiomyopathy-Related MutationsAbdelsayed Mena, Mercola Mark, Antzelevitch Charles
More abstracts from these authors:
Owens Anjali, Gao Weihua, Maksabedian Hernandez Ervant, Dubey Anand, Stevens Warren, Davis Matthew, Schuler Patricia, Pandya Manish, Han Eileen
Long-term effectiveness and safety of mavacamten in a real-world, multi-center, global study: Preliminary results of COLLIGO-HCM from a diverse cohort in the United StatesMacnamara James, Orlandi Paula, Li Leanne, Pichardo Angel, Bilen Ozlem, Adler Arnon, Maor Elad, Bastiaenen Rachel, Gold Matthew, Maksabedian Hernandez Ervant, Han Xu, Schuler Patricia, Salazar-mendiguchia Joel