Final ID: MP1497
Small, Dense LDL-C and Conventional LDL-C Similarly Predict Cardiovascular Risk and Benefit of Alirocumab in Statin-Treated Patients With Recent Acute Coronary Syndrome
Methods: The analysis included 11,837 participants in the ODYSSEY OUTCOMES trial (NCT01663402) with recent ACS and LDL-C ≥70 mg/dL despite optimized statin treatment. At baseline prior to randomized treatment with the PCSK9 monoclonal antibody alirocumab (N=5917) or placebo (N=5920), sdLDL-C was measured using the Denka (Nigata, Japan) method on a Roche cobas autoanalyzer and LDL-C was calculated with the Friedewald formula. In the placebo group, natural cubic splines depicted the relationships of sdLDL-C, LDL-C, and their ratio to the risk of MACE (CV death, non-fatal myocardial infarction or ischemic stroke, hospitalization for unstable angina, and ischemia-driven coronary revascularization) and treatment hazard ratio (HR: alirocumab/placebo) as a function of sdLDL-C and LDL-C.
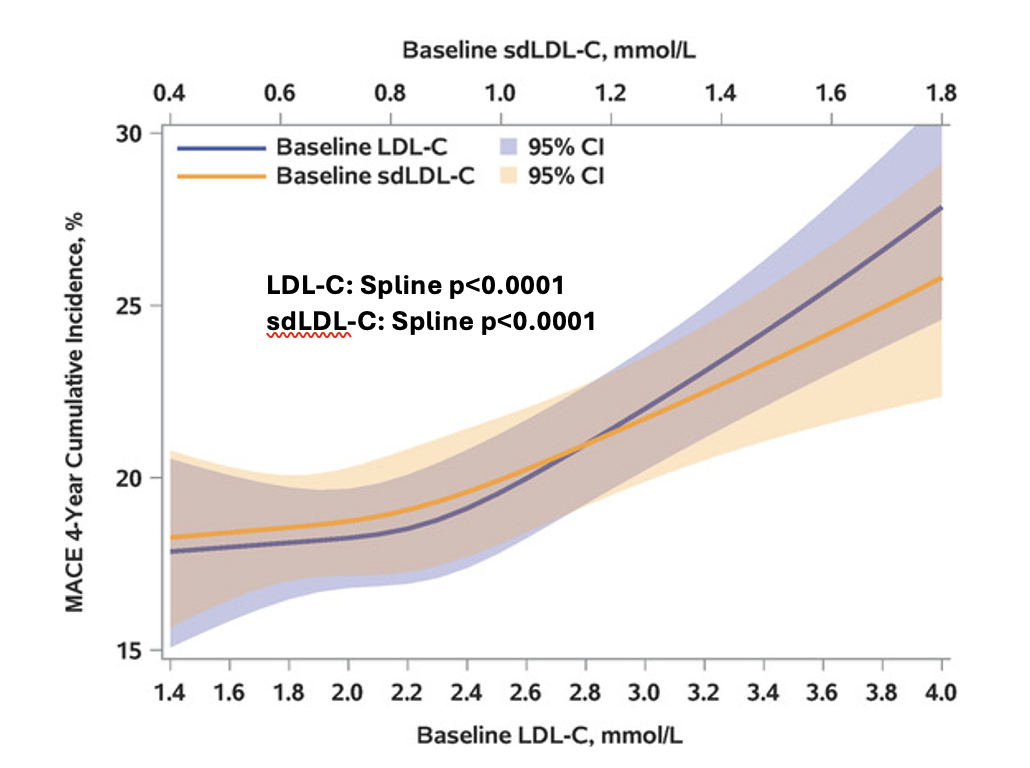
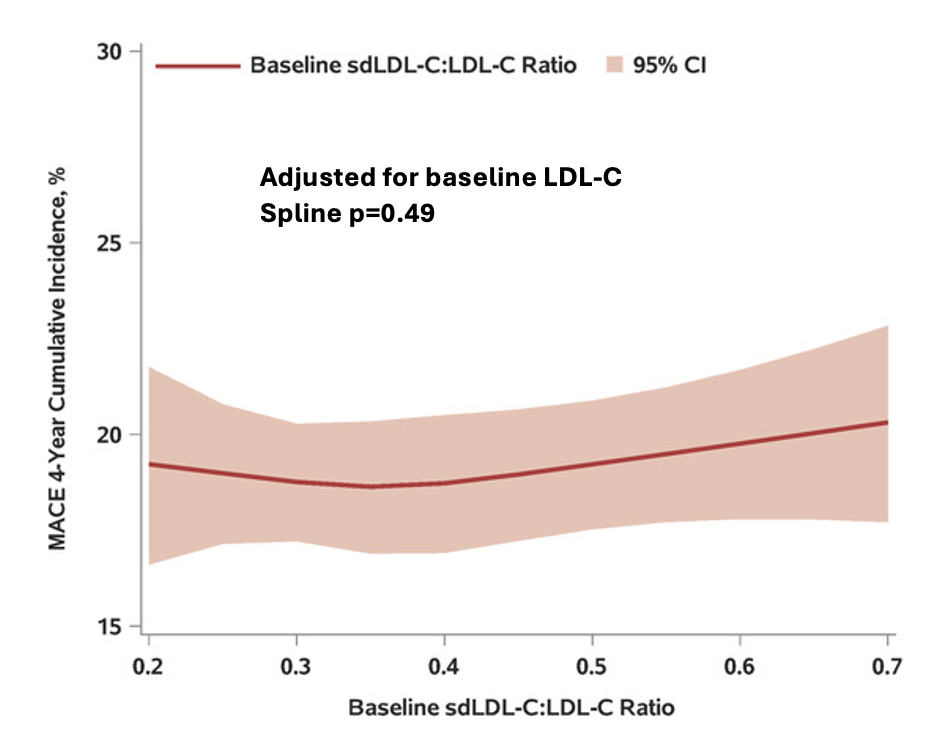
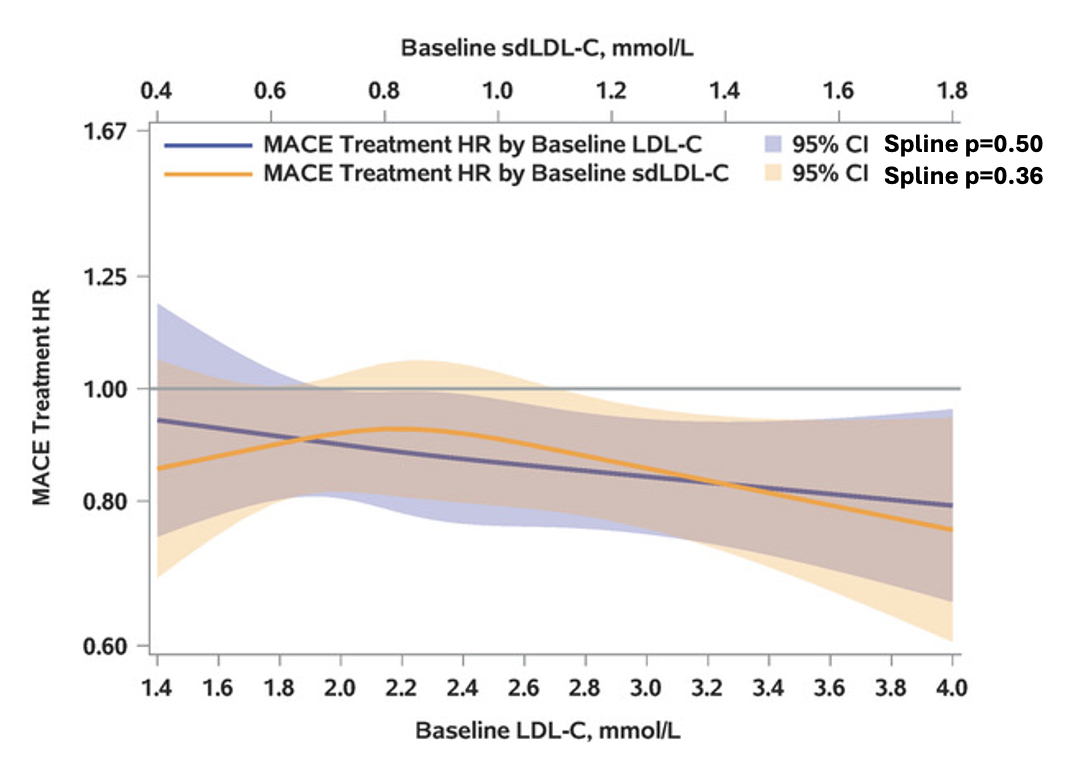
Results: In Figure Panel A, the risk of MACE in the placebo group increased with concentrations of baseline sdLDL-C and LDL-C, with nearly superimposable splines. In Panel B, the relationship of sdLDL-C/LDL-C to risk of MACE in the placebo group (adjusted for LDL-C) showed no evidence of greater risk with greater sdLDL-C fraction. Overall, alirocumab reduced the risk of MACE (HR 0.87, 95% CI 0.79, 0.95). Panel C shows that the treatment HR did not vary significantly across the range of either LDL-C or sdLDL-C.
Conclusion: In patients with recent ACS and LDL-C ≥70 mg/dL on optimized statin treatment, sdLDL-C and conventional LDL-C similarly predict risk of MACE and benefit of treatment with alirocumab. Measurement of sdLDL-C does not appear to provide additional prognostic or predictive information.
- Schwartz, Gregory ( University of Colorado School of Medicine , Aurora , Colorado , United States )
- Reijnders, Esther ( Leiden University Medical Center , Leiden , Netherlands )
- Romijn, Fred ( Leiden University Medical Center , Leiden , Netherlands )
- Stevanovic, Irena ( Sanofi , Paris , France )
- Tavori, Hagai ( Sanofi , Yakum , Israel )
- Van Neer, Nicolaas ( Leiden University Medical Center , Leiden , Netherlands )
- White, Harvey ( Auckland City Hospital , Auckland , New Zealand )
- Jukema, J ( Leiden University Medical Center , Leiden , Netherlands )
- Szarek, Michael ( CPC Clinical Research , Aurora , Colorado , United States )
- Cobbaert, Christa ( Leiden University Medical Center , Leiden , Netherlands )
- Ruhaak, Lucia Renee ( Leiden University Medical Center , Leiden , Netherlands )
- Schwertfeger, Markus ( Roche Diagnostics International Ltd , Rotkreuz , Switzerland )
- Bhatt, Deepak ( Mount Sinai Fuster Heart Hospital , New York , New York , United States )
- Bittner, Vera ( University of Alabama at Birmingham , Birmingham , Alabama , United States )
- Goodman, Shaun ( St Michaels Hospital , Toronto , Ontario , Canada )
- Harrington, Robert ( Weill Cornell Medicine , New York , New York , United States )
Meeting Info:
Session Info:
Cholesterol Chronicles: Lipid Markers and Cardiovascular Disease
Sunday, 11/09/2025 , 11:50AM - 01:05PM
Moderated Digital Poster Session
More abstracts on this topic:
Kamel Moaz, Arsanjani Reza, Awad Kamal, Mahmoud Ahmed K., Farina Juan, Scalia Isabel, Pereyra Milagros, Abbas Mohammed Tiseer, Baba Nima, Ayoub Chadi
4-Phenylbutyric Acid Reduces Endoplasmic Reticulum Retention and Partially Restores Function of LDLR p.D622N Mutation In Vitro: A Potential Therapy for HypercholesterolemiaWang Yongxiang, Zhang Piyi, Bai Ming, Zhang Zheng
More abstracts from these authors:
Tsimikas Sotirios, Garon Genevieve, Chong Yuan, Gong Xiaomin, Goodman Shaun, Harrington Robert, White Harvey, Zeiher Andreas, Steg Philippe, Schwartz Gregory, Szarek Michael, Cobbaert Christa, Reijnders Esther, Jukema J, Bhatt Deepak, Bittner Vera, Diaz Rafael, Fazio Sergio
Comparison of Lipoprotein(a) and Other Apo B Containing Lipoproteins as Predictors of Major Adverse Cardiovascular Events in ODYSSEY OUTCOMESBittner Vera, Goodman Shaun, Harrington Robert, White Harvey, Zeiher Andreas, Cobbaert Christa, Schwartz Gregory, Szarek Michael, Steg Philippe, Jukema J, Reijnders Esther, Bhatt Deepak, Diaz Rafael, Fazio Sergio, Garon Genevieve