Final ID: Sa4017
GLP-1 Agonist Therapy Attenuates Circulating Gut-Derived Trimethylamine N-Oxide (TMAO) and Improves Cardiac Function and Exercise Performance in Cardiometabolic HFpEF
Abstract Body (Do not enter title and authors here): Background: Heart failure with preserved ejection fraction (HFpEF) poses a significant global health burden, characterized by poorly understood and heterogeneous underlying mechanisms. Trimethylamine N-oxide (TMAO), a metabolite generated by gut microbiota contributes to cardiovascular diseases. Elevated circulating TMAO levels are associated with adverse cardiovascular events, including coronary artery disease, myocardial infarction, and HFpEF. However, the impact of current HFpEF treatments, such as glucagon-like peptide-1 receptor agonists (GLP-1 RAs), on TMAO levels and disease outcomes remains unknown.
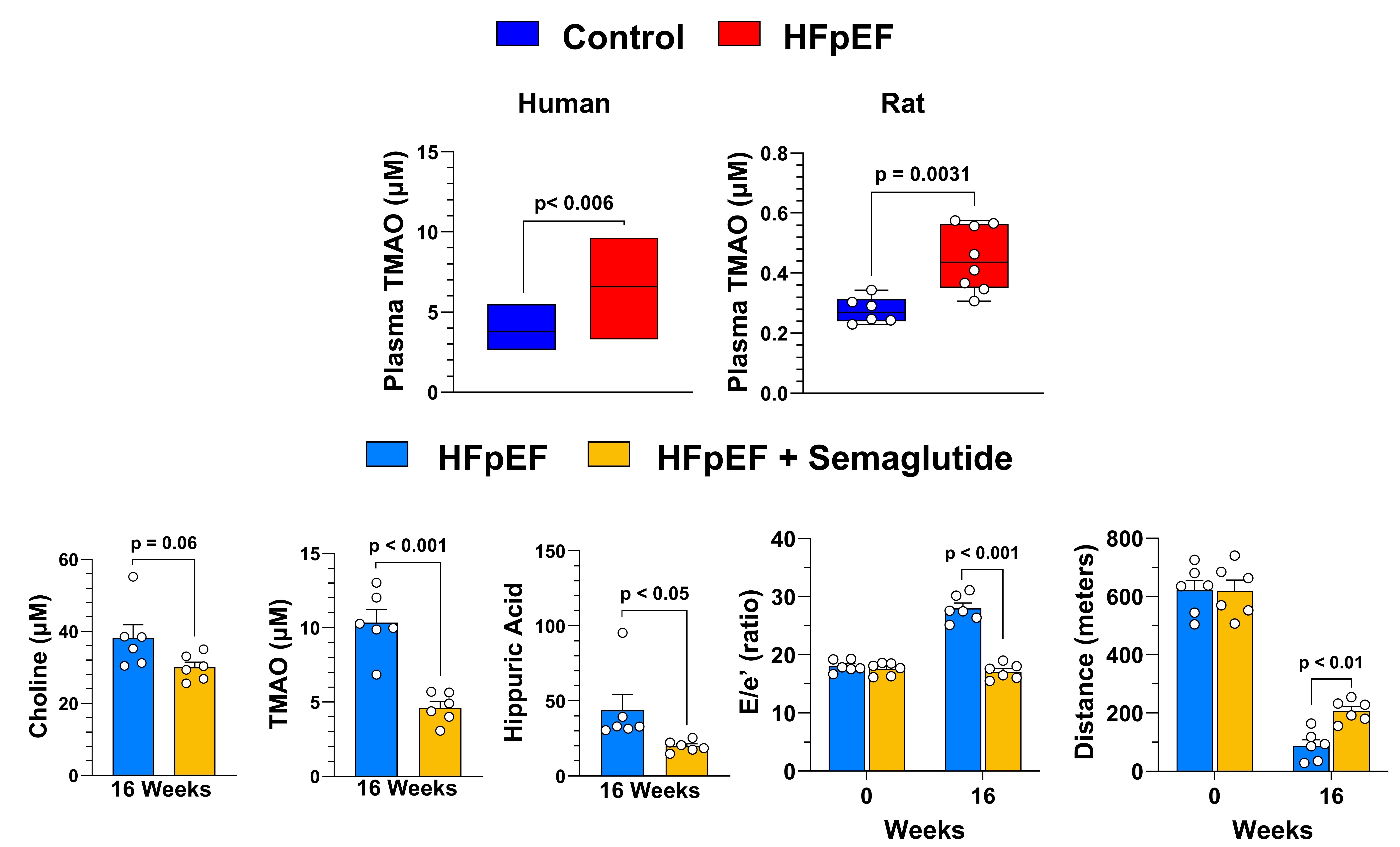
Methods: Plasma TMAO levels were quantified using LC-MS/MS in well-matched healthy controls and HFpEF patients (n=48 per group). These measurements were replicated in a preclinical model comparing WKY control rats and ZSF1 obese (Ob) HFpEF rats. Subsequently, male ZSF1 Ob rats (10 weeks old) were randomized to receive either vehicle (saline) or semaglutide (30 nmol/kg, subcutaneous, biweekly) for 16 weeks (n=6 per group). Comprehensive phenotyping in rats included echocardiography, treadmill exercise testing, invasive hemodynamic assessment, and histopathological analysis.
Results: HFpEF patients exhibited significantly elevated circulating TMAO levels compared to healthy controls. Similarly, ZSF1 Ob rats showed increased TMAO compared to WKY controls. Semaglutide treatment in ZSF1 Ob rats significantly reduced plasma TMAO, its precursor choline, and hippuric acid, while increasing lactoyl-phenylalanine levels. Functionally, semaglutide improved diastolic function (reduced E/e'), lowered left ventricular end-diastolic pressure (LVEDP), and improved treadmill exercise capacity. Also, semaglutide attenuated cardiac interstitial fibrosis and reduced intracardiac lipid accumulation.
Conclusion: Our findings reveal elevated TMAO levels in both HFpEF patients and a preclinical rat model of cardiometabolic HFpEF. Semaglutide treatment in HFpEF rats effectively reduced gut-derived TMAO and related metabolites while improving disease outcomes. These results suggest the beneficial effects of GLP-1 RA therapy in HFpEF involves remodeling of the gut microbiota-TMAO axis to attenuate gut dysbiosis and improve LV diastolic function and exercise capacity.
Methods: Plasma TMAO levels were quantified using LC-MS/MS in well-matched healthy controls and HFpEF patients (n=48 per group). These measurements were replicated in a preclinical model comparing WKY control rats and ZSF1 obese (Ob) HFpEF rats. Subsequently, male ZSF1 Ob rats (10 weeks old) were randomized to receive either vehicle (saline) or semaglutide (30 nmol/kg, subcutaneous, biweekly) for 16 weeks (n=6 per group). Comprehensive phenotyping in rats included echocardiography, treadmill exercise testing, invasive hemodynamic assessment, and histopathological analysis.
Results: HFpEF patients exhibited significantly elevated circulating TMAO levels compared to healthy controls. Similarly, ZSF1 Ob rats showed increased TMAO compared to WKY controls. Semaglutide treatment in ZSF1 Ob rats significantly reduced plasma TMAO, its precursor choline, and hippuric acid, while increasing lactoyl-phenylalanine levels. Functionally, semaglutide improved diastolic function (reduced E/e'), lowered left ventricular end-diastolic pressure (LVEDP), and improved treadmill exercise capacity. Also, semaglutide attenuated cardiac interstitial fibrosis and reduced intracardiac lipid accumulation.
Conclusion: Our findings reveal elevated TMAO levels in both HFpEF patients and a preclinical rat model of cardiometabolic HFpEF. Semaglutide treatment in HFpEF rats effectively reduced gut-derived TMAO and related metabolites while improving disease outcomes. These results suggest the beneficial effects of GLP-1 RA therapy in HFpEF involves remodeling of the gut microbiota-TMAO axis to attenuate gut dysbiosis and improve LV diastolic function and exercise capacity.
More abstracts on this topic:
A short version of HFD/L-NAME mouse model enabling time-effective proof of concept studies to evaluate drugs targeting the cardiometabolic and mild hypertension associated HFpEF phenotype.
Assaly Rana, Dubroca Caroline, Waget Aurelie, Perrier Kevin, Sulpice Thierry
Atherosclerotic features of plaque instability are transmitted via gut microbial transplantationNieri Riccardo, Pedicino Daniela, Russo Matteo, Liuzzo Giovanna, Limana Federica, Foglio Eleonora, De Maio Flavio, Severino Anna, Masucci Luca, D Aiello Alessia, Grimaldi Maria Chiara, Gervasoni Jacopo, Santoni Daniele