Final ID: Sa3001
Galectin-3 and sST2 for Early Risk Prediction in Pediatric Cardiac Failure Requiring Venoarterial Extracorporeal Membrane Oxygenation
Abstract Body (Do not enter title and authors here): Background
Galectin-3 and soluble suppression of tumorigenicity-2 (sST2) are biomarkers of fibrosis and inflammation, with prognostic value in adult cardiac failure both with and without mechanical circulatory support. Their utility in pediatric venoarterial extracorporeal membrane oxygenation (VA-ECMO), especially across different age, remains unclear.
Research Question
Can early Galectin-3 and sST2 levels, with or without age context, predict mortality in pediatric patients with cardiac failure requiring VA-ECMO?
Methods
We prospectively enrolled 34 pediatric patients on VA-ECMO. Plasma Galectin-3 and sST2 were measured from serial blood samples collected pre-cannulation, at 2-, 4-, and 6-hours post-cannulation, daily up to day 8, and at decannulation and the following day. Levels were quantified using ELISA. Biomarker trends were analyzed, using peak values from the first 3 days to compare outcomes between survivors (discharged) and non-survivors. Logistic regression models assessed the predictive performance of Galectin-3 and sST2 individually and in combination with age. Discrimination was evaluated using ROC curves and Youden’s Index.
Results
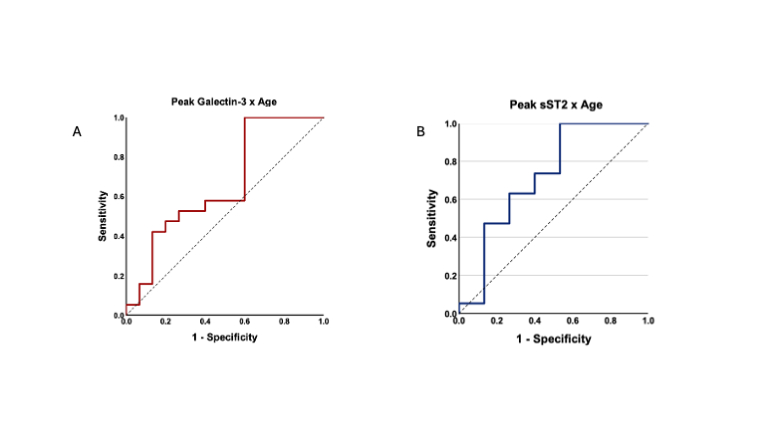
The cohort included 11 neonates (32%), 16 infants (47%), 5 children (15%), and 2 adolescents (6%). Thirteen patients (38%) were male. Diagnoses included extracorporeal cardiopulmonary resuscitation (38%), failure to wean from cardiopulmonary bypass (26%), cardiomyopathy/myocarditis (24%), and cardiac failure not otherwise specified (15%). Fifteen patients survived to discharge. The median duration of VA-ECMO support was longer in non-survivors (8 days [IQR 5–10]) compared to survivors (5 days [IQR 4–6.5]). Mean Galectin-3 levels were elevated early and declined over time but remained persistently higher in non-survivors. Mean sST2 peaked within 48 hours and declined in both groups, with a longer elevation in non-survivors. Individually, neither biomarker was predictive (AUC = 0.55 each). However, combining both with age improved discrimination (AUC = 0.73, p = 0.02). Optimal thresholds were identified: Galectin-3 ≥ 21.1 ng/mL and sST2 ≥ 1820.6 ng/mL.
Conclusion
Early serial sampling of Galectin-3 and sST2 revealed outcome-related temporal patterns. While neither marker alone was predictive, their combination with patient age improved prognostic model in younger patients. These findings support age-adjusted biomarker use for risk stratification in pediatric ECMO. Validation in larger cohorts is warranted.
Galectin-3 and soluble suppression of tumorigenicity-2 (sST2) are biomarkers of fibrosis and inflammation, with prognostic value in adult cardiac failure both with and without mechanical circulatory support. Their utility in pediatric venoarterial extracorporeal membrane oxygenation (VA-ECMO), especially across different age, remains unclear.
Research Question
Can early Galectin-3 and sST2 levels, with or without age context, predict mortality in pediatric patients with cardiac failure requiring VA-ECMO?
Methods
We prospectively enrolled 34 pediatric patients on VA-ECMO. Plasma Galectin-3 and sST2 were measured from serial blood samples collected pre-cannulation, at 2-, 4-, and 6-hours post-cannulation, daily up to day 8, and at decannulation and the following day. Levels were quantified using ELISA. Biomarker trends were analyzed, using peak values from the first 3 days to compare outcomes between survivors (discharged) and non-survivors. Logistic regression models assessed the predictive performance of Galectin-3 and sST2 individually and in combination with age. Discrimination was evaluated using ROC curves and Youden’s Index.
Results
The cohort included 11 neonates (32%), 16 infants (47%), 5 children (15%), and 2 adolescents (6%). Thirteen patients (38%) were male. Diagnoses included extracorporeal cardiopulmonary resuscitation (38%), failure to wean from cardiopulmonary bypass (26%), cardiomyopathy/myocarditis (24%), and cardiac failure not otherwise specified (15%). Fifteen patients survived to discharge. The median duration of VA-ECMO support was longer in non-survivors (8 days [IQR 5–10]) compared to survivors (5 days [IQR 4–6.5]). Mean Galectin-3 levels were elevated early and declined over time but remained persistently higher in non-survivors. Mean sST2 peaked within 48 hours and declined in both groups, with a longer elevation in non-survivors. Individually, neither biomarker was predictive (AUC = 0.55 each). However, combining both with age improved discrimination (AUC = 0.73, p = 0.02). Optimal thresholds were identified: Galectin-3 ≥ 21.1 ng/mL and sST2 ≥ 1820.6 ng/mL.
Conclusion
Early serial sampling of Galectin-3 and sST2 revealed outcome-related temporal patterns. While neither marker alone was predictive, their combination with patient age improved prognostic model in younger patients. These findings support age-adjusted biomarker use for risk stratification in pediatric ECMO. Validation in larger cohorts is warranted.
More abstracts on this topic:
β1-adrenergic autoantibodies (β1-AA) augment macropinocytosis in CD4+ T cells, leading to the expansion of CD4+CD28− T cell subsets in heart failure.
Sun Fei, Yao Junyan, Li Bingjie, Zhang Suli, Liu Huirong
A Novel Echocardiography Risk Score Predicted Mortality In Patients With Heart Failure With Preserved Ejection Fraction.Iwakura Katsuomi, Yoshio Yasumura, Hikoso Shungo, Okada Katsuki, Nakatani Daisaku, Sotomi Yohei, Sakata Yasushi, Tanaka Nobuaki, Okada Masato, Okamura Atsunori, Heitaro Watanabe, Seo Masahiro, Hayashi Takaharu, Yano Masamichi, Yamada Takahisa