Final ID: 4359302
Incidence, Risk Factors, and Outcomes in Triggered Atrial Fibrillation: Insights from the VITAL-AF Trial
Abstract Body (Do not enter title and authors here): Introduction
Triggered atrial fibrillation (AF), defined as new-onset AF in the setting of a reversible physiological stressor, is common. However, risk factors, outcomes, and management remain poorly understood.
Methods
We analyzed data from VITAL-AF, a pragmatic, cluster-randomized AF screening trial (2018-2019) of adults aged 65 and greater across 16 primary care practices affiliated with Massachusetts General Hospital, with 2 years of follow-up and adjudicated incident AF type (triggered vs. primary AF) and clinical outcomes. We compared associations between clinical AF risk factors and incident AF type (i.e, triggered vs non-triggered/primary) using Fine-Gray models. We quantified rates of OAC initiation following AF diagnosis. We then fit Cox proportional hazards models to measure the association between AF type and a composite endpoint of major bleeding, stroke, and all-cause mortality (pre-specified adjudicated VITAL-AF outcomes), with adjustment for CHA2DS2-VASc (stroke) and ATRIA (bleeding) scores and time-varying OAC exposure.
Results
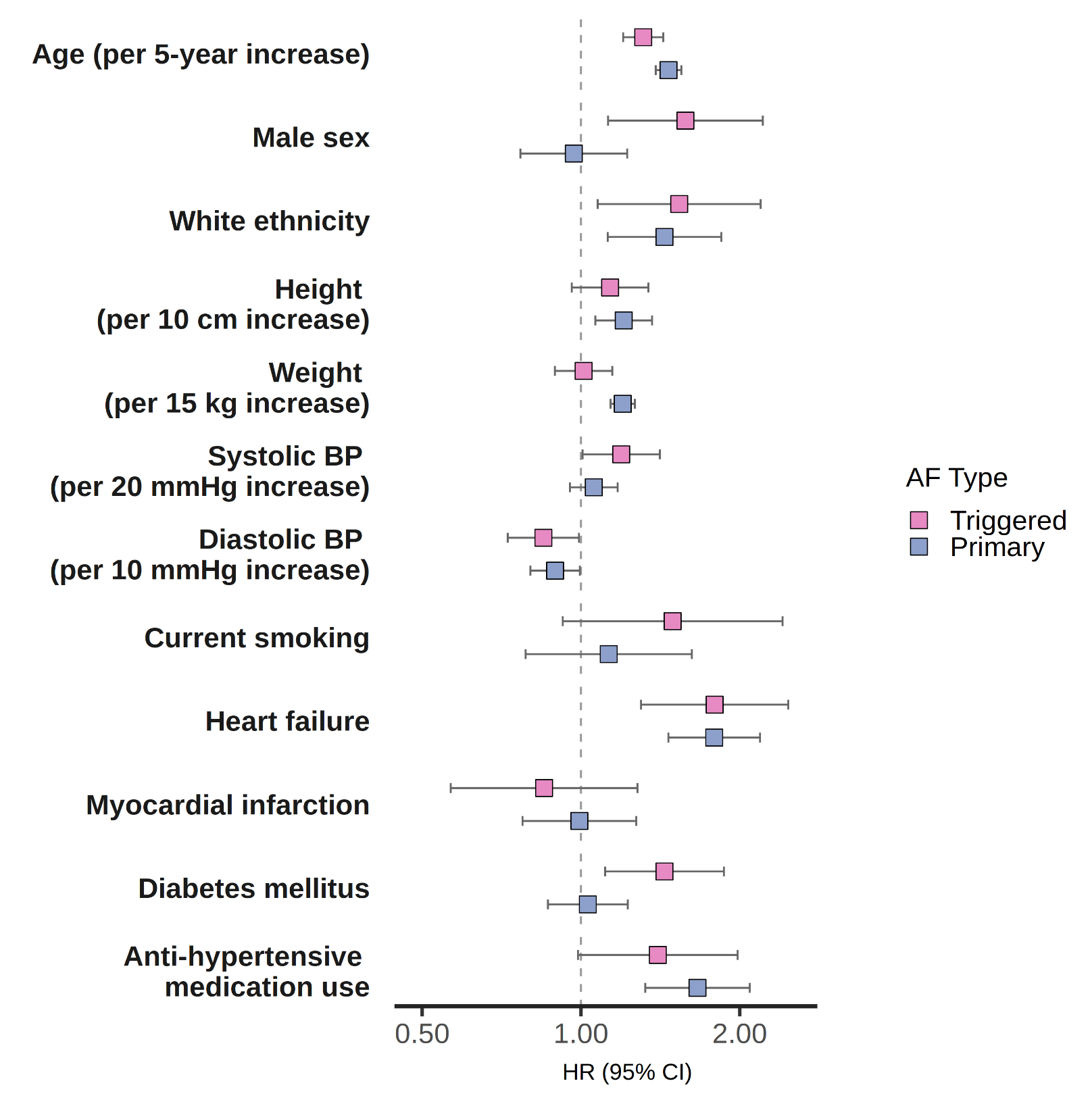
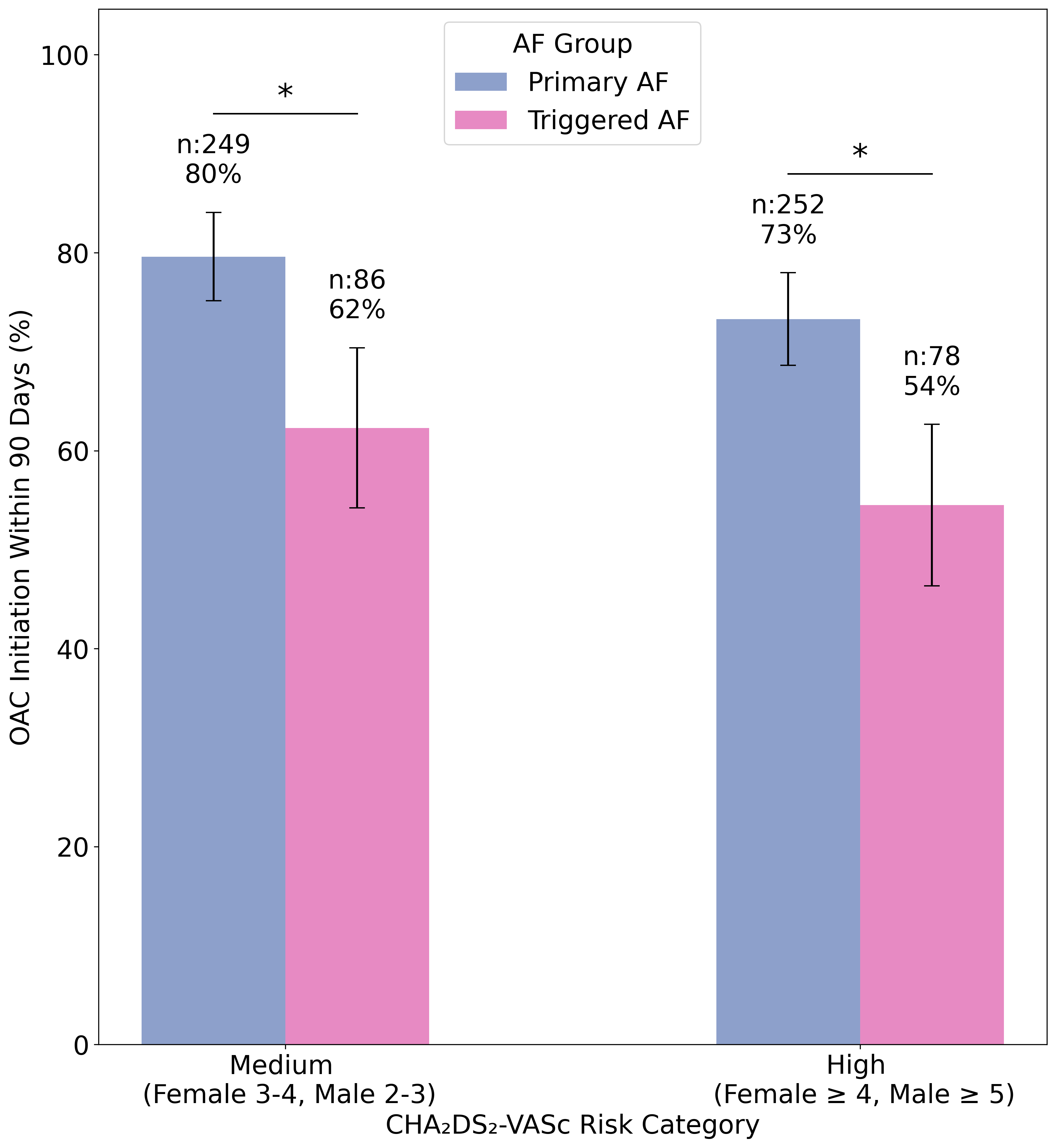
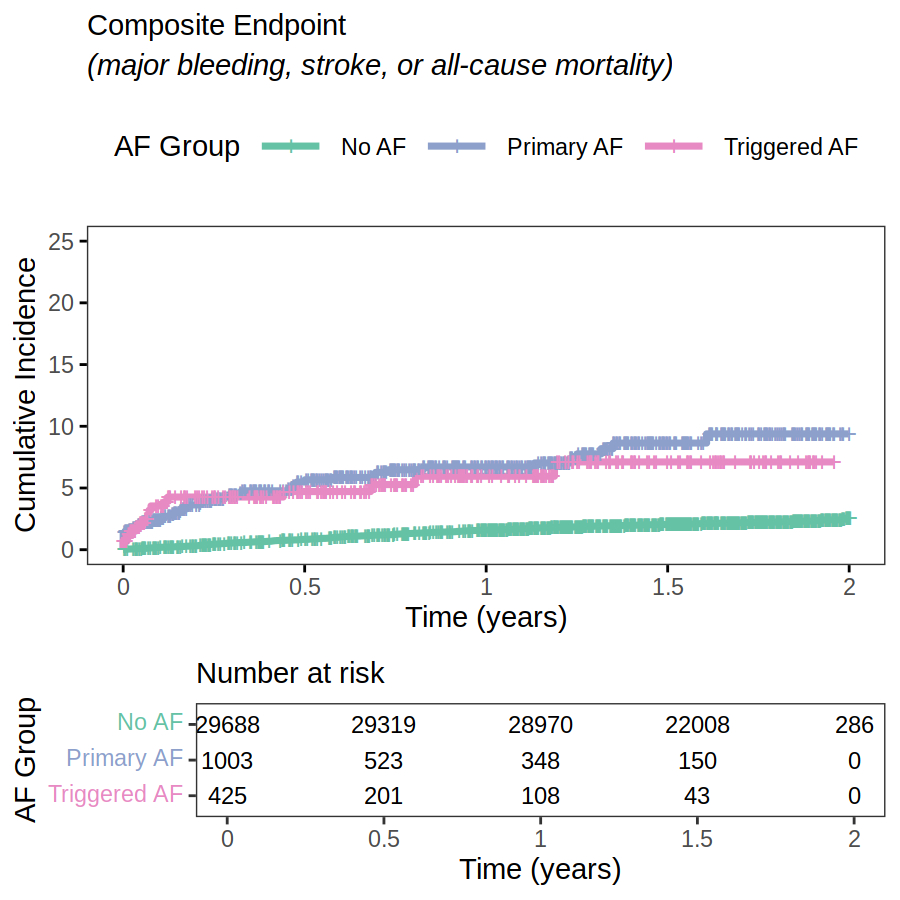
The study included 30,215 patients (59% female, 83% White, mean age 74). Of 998 incident AF events, 290 (29%) were triggered AF. Clinical risk factors including age, hypertension, and heart failure showed similar associations with both primary and triggered AF (Fig. 1). OAC initiation within 90 days of diagnosis was lower for triggered AF than primary AF, notably in medium (63% vs. 79%) and high (54% vs. 74%) CHA2DS2-VASc risk groups (p<0.05 for both) (Fig. 2). The incidence of the composite endpoint (per 100 person years) was 1.32 (95% CI 1.22-1.43), 8.06 (6.03-10.54), and 7.45 (4.42-11.78), for no AF, primary AF, and triggered AF, respectively (Fig. 3). In adjusted models, both primary (HR 2.23; 95% CI, 1.45-3.43) and triggered AF (HR 2.39; 95% CI, 1.36-4.22) were associated with similarly higher risk of the composite endpoint compared to no AF.
Conclusions
Among over 30,000 primary care patients with manually adjudicated events, nearly one-third of incident AF cases were triggered. Despite similar risk factor profiles and similarly higher risk of AF-related adverse outcomes, OAC use appears lower when AF is triggered. Future work is needed to raise awareness of the substantial morbidity and mortality associated with triggered atrial fibrillation and to develop standardized approaches to risk stratification and anticoagulation in this population.
Triggered atrial fibrillation (AF), defined as new-onset AF in the setting of a reversible physiological stressor, is common. However, risk factors, outcomes, and management remain poorly understood.
Methods
We analyzed data from VITAL-AF, a pragmatic, cluster-randomized AF screening trial (2018-2019) of adults aged 65 and greater across 16 primary care practices affiliated with Massachusetts General Hospital, with 2 years of follow-up and adjudicated incident AF type (triggered vs. primary AF) and clinical outcomes. We compared associations between clinical AF risk factors and incident AF type (i.e, triggered vs non-triggered/primary) using Fine-Gray models. We quantified rates of OAC initiation following AF diagnosis. We then fit Cox proportional hazards models to measure the association between AF type and a composite endpoint of major bleeding, stroke, and all-cause mortality (pre-specified adjudicated VITAL-AF outcomes), with adjustment for CHA2DS2-VASc (stroke) and ATRIA (bleeding) scores and time-varying OAC exposure.
Results
The study included 30,215 patients (59% female, 83% White, mean age 74). Of 998 incident AF events, 290 (29%) were triggered AF. Clinical risk factors including age, hypertension, and heart failure showed similar associations with both primary and triggered AF (Fig. 1). OAC initiation within 90 days of diagnosis was lower for triggered AF than primary AF, notably in medium (63% vs. 79%) and high (54% vs. 74%) CHA2DS2-VASc risk groups (p<0.05 for both) (Fig. 2). The incidence of the composite endpoint (per 100 person years) was 1.32 (95% CI 1.22-1.43), 8.06 (6.03-10.54), and 7.45 (4.42-11.78), for no AF, primary AF, and triggered AF, respectively (Fig. 3). In adjusted models, both primary (HR 2.23; 95% CI, 1.45-3.43) and triggered AF (HR 2.39; 95% CI, 1.36-4.22) were associated with similarly higher risk of the composite endpoint compared to no AF.
Conclusions
Among over 30,000 primary care patients with manually adjudicated events, nearly one-third of incident AF cases were triggered. Despite similar risk factor profiles and similarly higher risk of AF-related adverse outcomes, OAC use appears lower when AF is triggered. Future work is needed to raise awareness of the substantial morbidity and mortality associated with triggered atrial fibrillation and to develop standardized approaches to risk stratification and anticoagulation in this population.
More abstracts on this topic:
A Multi-Center Clinic Site Comparison of Patient-level factors Affecting Oral Anticoagulation Prescription for Atrial Fibrillation
Iqbal Fatima, Hoang Kenneth, Chiadika Simbo
A Deep Learning Topic Analysis Approach for Enhancing Risk Assessment in Heart Failure Using Unstructured Clinical NotesAdejumo Philip, Pedroso Aline, Khera Rohan