Final ID: MP2215
Glucagon-Like Peptide-1 Receptor Agonists Show Varied Impact on Venous Thromboembolism Risk: A Comprehensive Bayesian Network Meta-Analysis of Randomized Controlled Trials
Abstract Body (Do not enter title and authors here): Background: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are increasingly used in diabetes management, but their association with venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), remains unclear.
Research Questions: We sought to determine whether glucagon-like peptide-1 receptor agonists (GLP-1RAs) significantly increase venous thromboembolism (VTE) risk compared to placebo or other anti-diabetic drugs.
Methods: A systematic search of PubMed, SCOPUS, and EMBASE was conducted for RCTs comparing GLP-1RAs (dulaglutide, lixisenatide, exenatide, semaglutide, albiglutide, liraglutide) with placebo or other anti-diabetic drugs, reporting DVT and PE outcomes. A Bayesian network meta-analysis was performed to estimate odds ratios (OR) with 95% credible intervals (CrI) using Markov Chain Monte Carlo (MCMC) methods, and convergence was evaluated through the Gelman-Rubin diagnostic. Surface under the cumulative ranking curve (SUCRA) ranked treatments by efficacy.
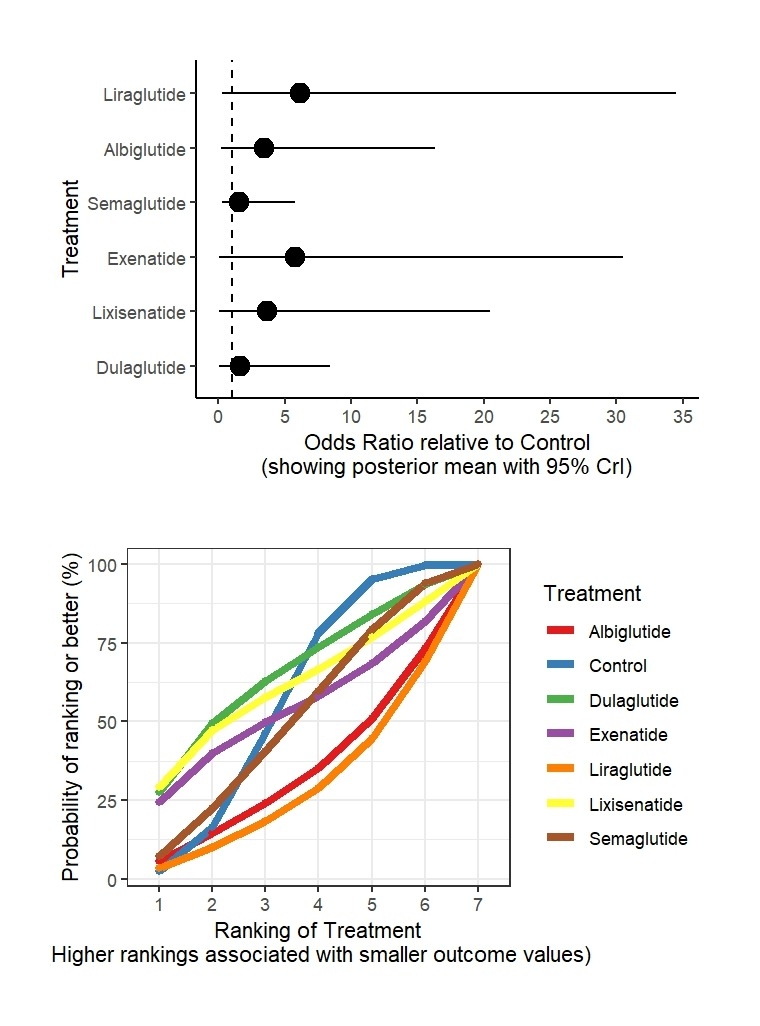
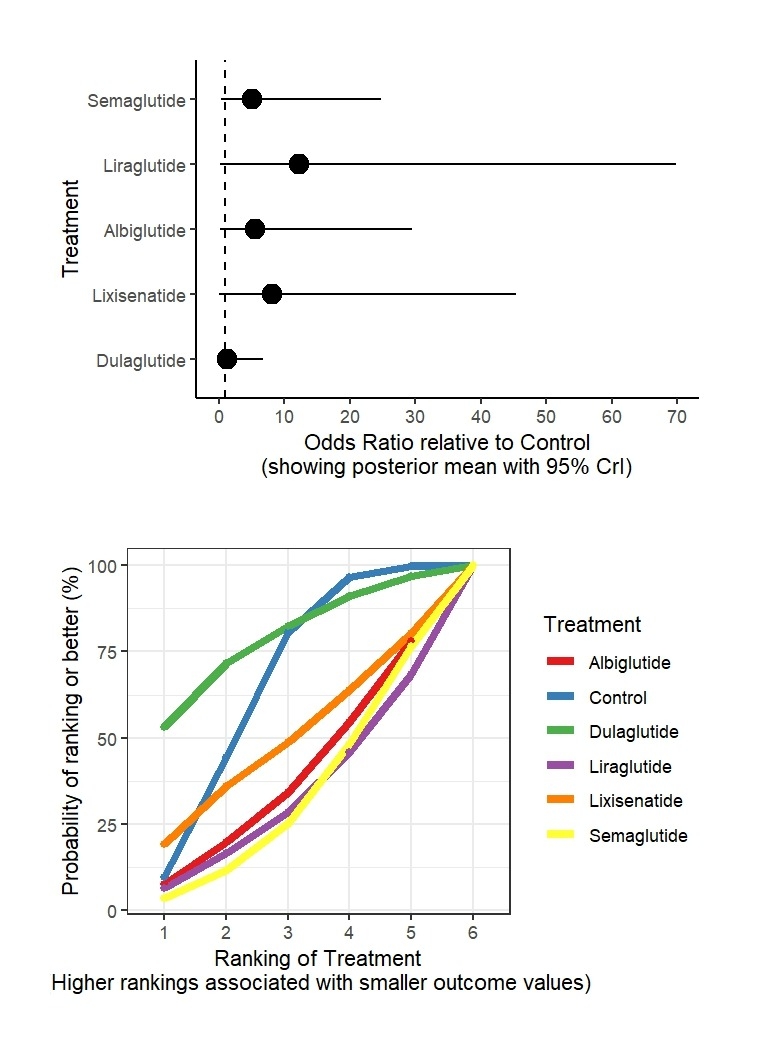
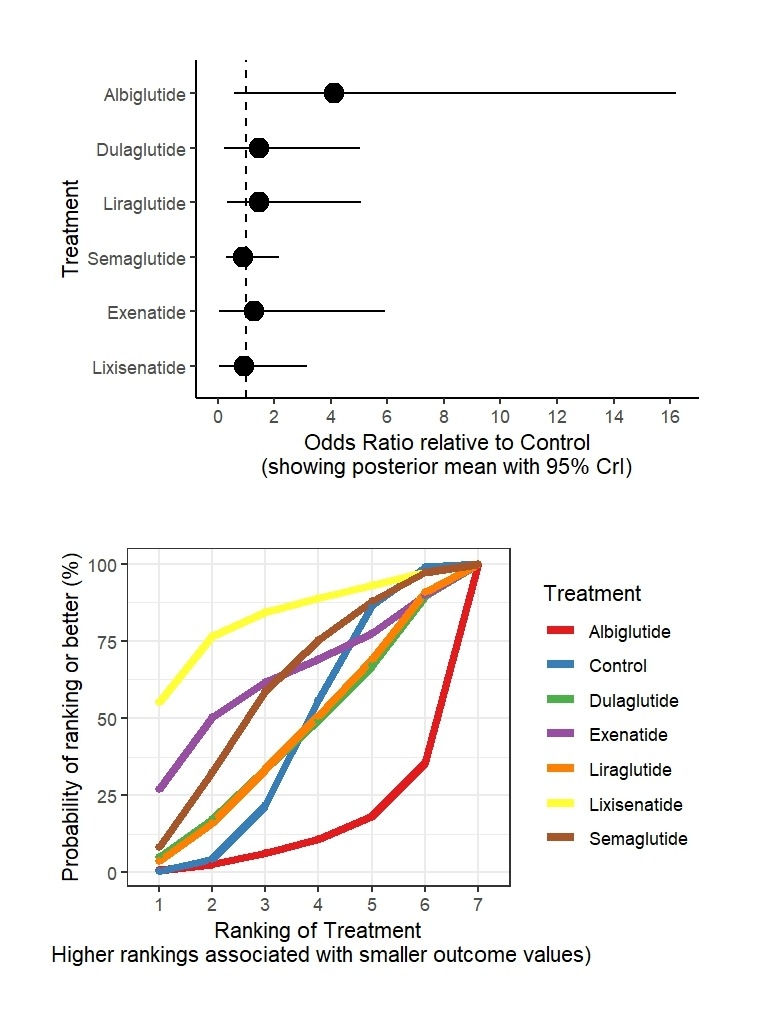
Results: From 39 RCTs with 70,499 participants, GLP-1RAs showed varied risks. For VTE versus control: dulaglutide (OR: 1.63, 95% CrI: 0.05 to 8.45; SUCRA: 65.23%), lixisenatide (OR: 3.66, 95% CrI: 0.03 to 20.47; SUCRA: 61.02%), exenatide (OR: 5.75, 95% CrI: 0.03 to 30.48; SUCRA: 53.77%), semaglutide (OR: 1.59, 95% CrI: 0.28 to 5.82; SUCRA: 50.51%), albiglutide (OR: 3.48, 95% CrI: 0.19 to 16.31; SUCRA: 33.95%), liraglutide (OR: 6.15, 95% CrI: 0.31 to 34.48; SUCRA: 29.15%), control (SUCRA: 56.38%). For DVT versus control: dulaglutide (OR: 1.24, 95% CrI: 0.02 to 6.82; SUCRA: 79.04%), lixisenatide (OR: 8.08, 95% CrI: 0.05 to 45.38; SUCRA: 49.66%), albiglutide (OR: 5.49, 95% CrI: 0.19 to 29.58; SUCRA: 38.77%), liraglutide (OR: 12.28, 95% CrI: 0.21 to 69.73; SUCRA: 33.24%), semaglutide (OR: 5.11, 95% CrI: 0.41 to 24.78; SUCRA: 33.07%), control (SUCRA: 66.23%). For PE versus control: lixisenatide (OR: 0.94, 95% CrI: 0.03 to 3.15; SUCRA: 82.64%), exenatide (OR: 1.28, 95% CrI: 0.05 to 5.91; SUCRA: 62.62%), semaglutide (OR: 0.88, 95% CrI: 0.28 to 2.15; SUCRA: 60.08%), liraglutide (OR: 1.45, 95% CrI: 0.33 to 5.06; SUCRA: 43.99%), dulaglutide (OR: 1.48, 95% CrI: 0.24 to 5.03; SUCRA: 43.58%), control (SUCRA: 44.71%), albiglutide (OR: 4.10, 95% CrI: 0.57 to 16.20; SUCRA: 12.38%).
Conclusions: GLP-1RAs exhibit varied VTE risks, with liraglutide showing the highest risk for VTE, and albiglutide for PE. Dulaglutide and lixisenatide appear safer for DVT and PE, respectively.
Research Questions: We sought to determine whether glucagon-like peptide-1 receptor agonists (GLP-1RAs) significantly increase venous thromboembolism (VTE) risk compared to placebo or other anti-diabetic drugs.
Methods: A systematic search of PubMed, SCOPUS, and EMBASE was conducted for RCTs comparing GLP-1RAs (dulaglutide, lixisenatide, exenatide, semaglutide, albiglutide, liraglutide) with placebo or other anti-diabetic drugs, reporting DVT and PE outcomes. A Bayesian network meta-analysis was performed to estimate odds ratios (OR) with 95% credible intervals (CrI) using Markov Chain Monte Carlo (MCMC) methods, and convergence was evaluated through the Gelman-Rubin diagnostic. Surface under the cumulative ranking curve (SUCRA) ranked treatments by efficacy.
Results: From 39 RCTs with 70,499 participants, GLP-1RAs showed varied risks. For VTE versus control: dulaglutide (OR: 1.63, 95% CrI: 0.05 to 8.45; SUCRA: 65.23%), lixisenatide (OR: 3.66, 95% CrI: 0.03 to 20.47; SUCRA: 61.02%), exenatide (OR: 5.75, 95% CrI: 0.03 to 30.48; SUCRA: 53.77%), semaglutide (OR: 1.59, 95% CrI: 0.28 to 5.82; SUCRA: 50.51%), albiglutide (OR: 3.48, 95% CrI: 0.19 to 16.31; SUCRA: 33.95%), liraglutide (OR: 6.15, 95% CrI: 0.31 to 34.48; SUCRA: 29.15%), control (SUCRA: 56.38%). For DVT versus control: dulaglutide (OR: 1.24, 95% CrI: 0.02 to 6.82; SUCRA: 79.04%), lixisenatide (OR: 8.08, 95% CrI: 0.05 to 45.38; SUCRA: 49.66%), albiglutide (OR: 5.49, 95% CrI: 0.19 to 29.58; SUCRA: 38.77%), liraglutide (OR: 12.28, 95% CrI: 0.21 to 69.73; SUCRA: 33.24%), semaglutide (OR: 5.11, 95% CrI: 0.41 to 24.78; SUCRA: 33.07%), control (SUCRA: 66.23%). For PE versus control: lixisenatide (OR: 0.94, 95% CrI: 0.03 to 3.15; SUCRA: 82.64%), exenatide (OR: 1.28, 95% CrI: 0.05 to 5.91; SUCRA: 62.62%), semaglutide (OR: 0.88, 95% CrI: 0.28 to 2.15; SUCRA: 60.08%), liraglutide (OR: 1.45, 95% CrI: 0.33 to 5.06; SUCRA: 43.99%), dulaglutide (OR: 1.48, 95% CrI: 0.24 to 5.03; SUCRA: 43.58%), control (SUCRA: 44.71%), albiglutide (OR: 4.10, 95% CrI: 0.57 to 16.20; SUCRA: 12.38%).
Conclusions: GLP-1RAs exhibit varied VTE risks, with liraglutide showing the highest risk for VTE, and albiglutide for PE. Dulaglutide and lixisenatide appear safer for DVT and PE, respectively.
More abstracts on this topic:
A Real-world Evaluation of Longitudinal Healthcare Expenses in a Health System Registry of Type-2 Diabetes Mellitus and Cardiovascular Disease Enabled by the 21st Century Cures Act
Dhingra Lovedeep, Aminorroaya Arya, Pedroso Aline, Rajpura Jigar, Mehanna Sherif, Tonnu-mihara Ivy, Khera Rohan
Add-on Therapy with Dantrolene, a RyR2 Stabilizer, Terminates Ventricular Tachycardia Storm refractory to Intravenous Amiodarone in Heart Failure.Nawata Junya, Omuro Ayumi, Fukuda Masakazu, Suetomi Takeshi, Miyazaki Yosuke, Fujimura Tatsuhiro, Mochizuki Mamoru, Sano Motoaki, Kobayashi Shigeki, Ishikawa Maho, Nakata Yuki, Murakawa Kaori, Nakashima Yusuke, Hisaoka Masahiro, Matsuyama Tetsuya, Nakamura Yoshihide