Final ID: 4357419
Multidomain Risk Profiles and Disease Progression Across Atherosclerotic Vascular Beds
Abstract Body (Do not enter title and authors here): Background: Atherosclerotic cardiovascular disease (ASCVD) manifests variably across coronary (CAD), cerebrovascular (CVD), and peripheral artery (PAD) beds. The contributions of sociodemographic, clinical, lifestyle, and genetic domains to ASCVD onset, localization, and progression remain incompletely understood.
Objectives: To (1) evaluate how multidomain risk factors relate to ASCVD onset by vascular bed; (2) identify predictors associated with ASCVD localization; and (3) determine key drivers of progression from single- to multibed ASCVD.
Methods: We analyzed 468,266 UK Biobank participants without baseline ASCVD. Risk factor importance was assessed using multivariable Cox models and machine learning–derived SHapley Additive Explanations (SHAP). Age-varying hazard ratios were estimated. Multinomial logistic regression evaluated disease localization, and discrete-time multistate models examined transitions to single-bed, multibed, and fatal states. Analyses used ten multiple imputed datasets.
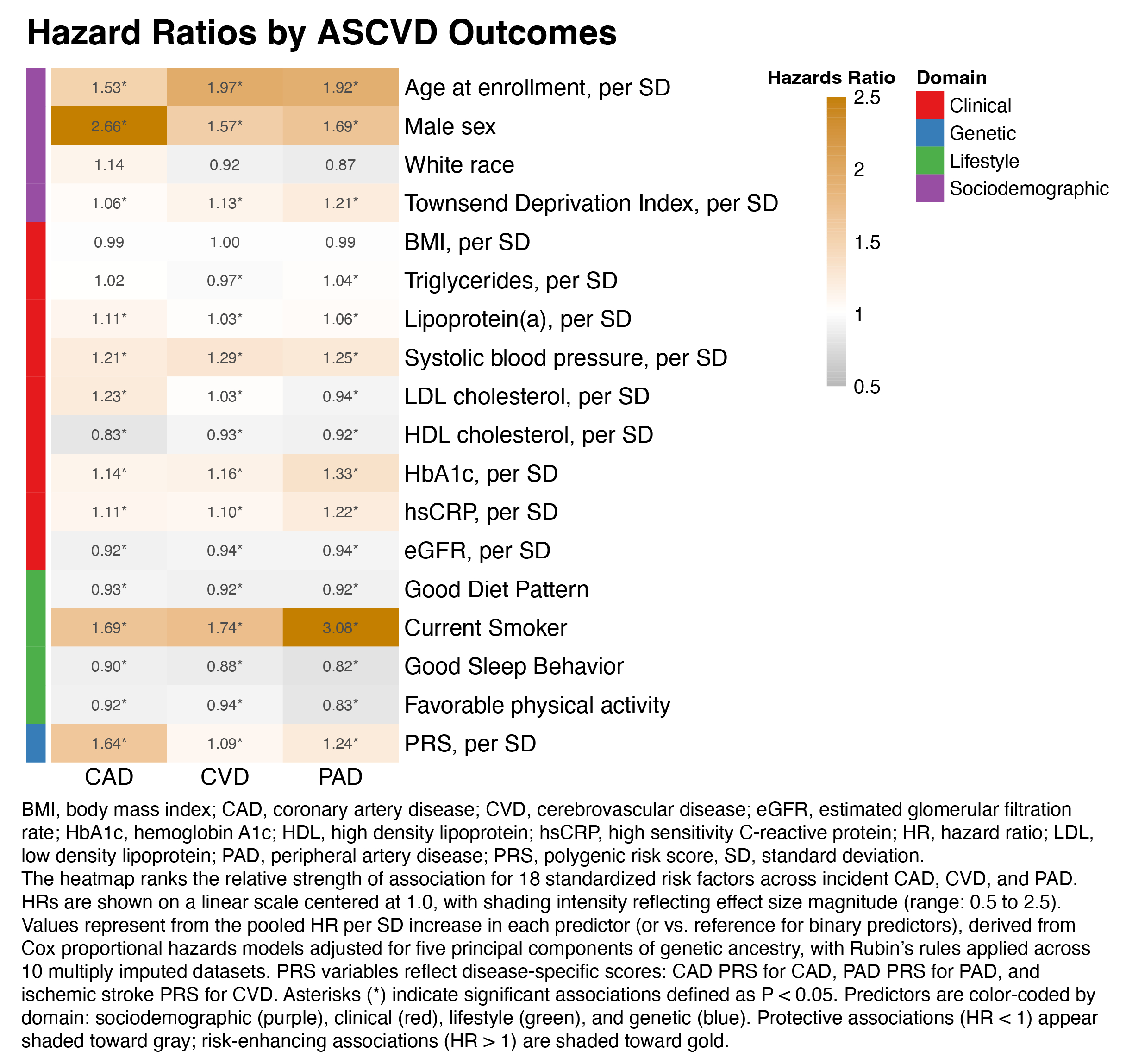
Results: Risk factor architecture varied by vascular bed (Fig. 1). PAD onset was dominated by inflammation and metabolic dysfunction—particularly C-reactive protein (CRP), HbA1c, and smoking (e.g., CRP hazard ratio [HR] 1.22, 95% CI 1.19–1.26)—while CAD was more strongly linked to low-density lipoprotein cholesterol (LDL-C; HR 1.23, 95% CI 1.21–1.25), the CAD polygenic risk score (PRS), and systolic blood pressure (SBP). CVD shared features with both PAD and CAD, with contributions from CRP, SBP, and socioeconomic deprivation. Healthy sleep and physical activity were protective. SHAP analyses confirmed these vascular bed–specific profiles. Age-varying models showed stronger effects of PRSs and clinical predictors before age 60. In multinomial models (Fig. 2), non-CAD phenotypes were more associated with CRP, HbA1c, and smoking, whereas CAD was more predicted by LDL-C and CAD PRS. Smoking showed the largest differential effect (odds ratio [OR] PAD vs CAD 2.09, 95% CI 1.93–2.26). Multistate models (Fig. 3) showed CRP, HbA1c, SBP, and CAD PRS predicted progression to multibed ASCVD, while transitions to death were driven by metabolic, renal, and inflammatory markers.
Conclusions: ASCVD risk factors differ in relative importance across vascular beds and stages. These findings underscore the need for vascular bed–specific prevention strategies that integrate early genetic risk stratification with aggressive management of modifiable factors across the disease continuum.
Objectives: To (1) evaluate how multidomain risk factors relate to ASCVD onset by vascular bed; (2) identify predictors associated with ASCVD localization; and (3) determine key drivers of progression from single- to multibed ASCVD.
Methods: We analyzed 468,266 UK Biobank participants without baseline ASCVD. Risk factor importance was assessed using multivariable Cox models and machine learning–derived SHapley Additive Explanations (SHAP). Age-varying hazard ratios were estimated. Multinomial logistic regression evaluated disease localization, and discrete-time multistate models examined transitions to single-bed, multibed, and fatal states. Analyses used ten multiple imputed datasets.
Results: Risk factor architecture varied by vascular bed (Fig. 1). PAD onset was dominated by inflammation and metabolic dysfunction—particularly C-reactive protein (CRP), HbA1c, and smoking (e.g., CRP hazard ratio [HR] 1.22, 95% CI 1.19–1.26)—while CAD was more strongly linked to low-density lipoprotein cholesterol (LDL-C; HR 1.23, 95% CI 1.21–1.25), the CAD polygenic risk score (PRS), and systolic blood pressure (SBP). CVD shared features with both PAD and CAD, with contributions from CRP, SBP, and socioeconomic deprivation. Healthy sleep and physical activity were protective. SHAP analyses confirmed these vascular bed–specific profiles. Age-varying models showed stronger effects of PRSs and clinical predictors before age 60. In multinomial models (Fig. 2), non-CAD phenotypes were more associated with CRP, HbA1c, and smoking, whereas CAD was more predicted by LDL-C and CAD PRS. Smoking showed the largest differential effect (odds ratio [OR] PAD vs CAD 2.09, 95% CI 1.93–2.26). Multistate models (Fig. 3) showed CRP, HbA1c, SBP, and CAD PRS predicted progression to multibed ASCVD, while transitions to death were driven by metabolic, renal, and inflammatory markers.
Conclusions: ASCVD risk factors differ in relative importance across vascular beds and stages. These findings underscore the need for vascular bed–specific prevention strategies that integrate early genetic risk stratification with aggressive management of modifiable factors across the disease continuum.
More abstracts on this topic:
Association between Neurological Complications and Carotid-cerebral Artery Disease after Coronary Artery Bypass Grafting
Liu Yi, Zhu Yunpeng, Jin Wei, Jin Zhijia, Gu Jianwei, Zhao Qiang
ADC-based Infarct Density – Validating a Novel Imaging Biomarker of Functional Outcome after Endovascular ThrombectomyFavilla Christopher, Bonkhoff Anna, Rost Natalia, Messe Steven, Regenhardt Robert, Denny Braden, Simonsen Claus, Shakibajahromi Banafsheh, Patel Aman, Leslie-mazwi Thabele, Dmytriw Adam, Schirmer Markus