Final ID: LB20
Atorvastatin Treatment and Rebleeding in Cerebral Cavernous Malformations: A Randomized, Placebo-Controlled, Double-Blinded Clinical Trial
Methods. The trial was powered to detect an absolute difference of 20% in the mean percent change in lesional QSM per year (2-tailed, power 0.9, alpha 0.05), accounting for patient attrition, with sample size recalculation at trial midpoint. Eighty subjects were randomized 1-1 to atorvastatin or placebo, and 64 contributed at least one annual paired QSM assessment, exceeding the sample size needed, all with > 90% active drug compliance.
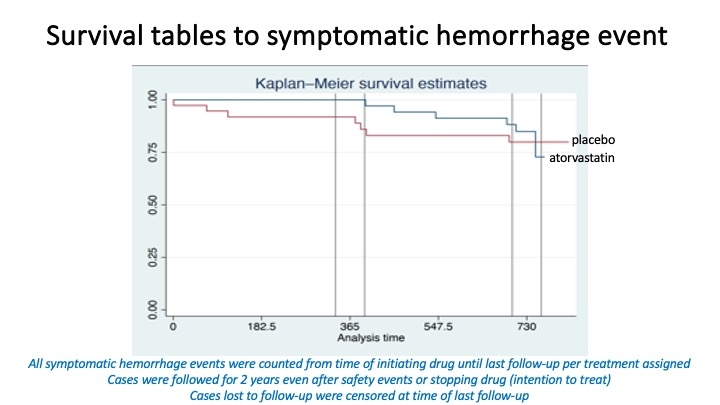
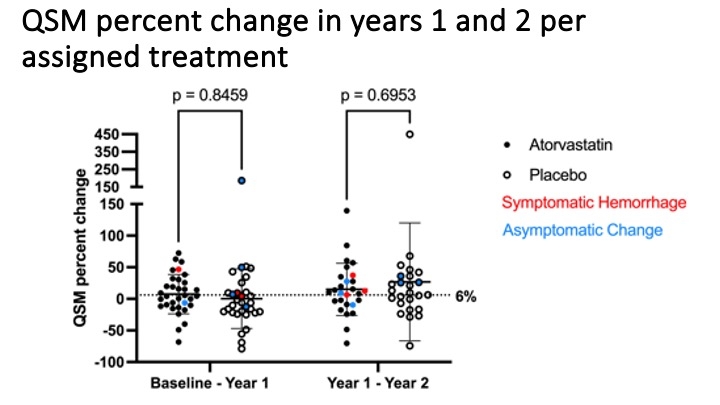
Findings. The mean annual lesional QSM percent change in sex adjusted mixed model averaged over 2 years was 10.9 (95% CI -3.2 to +25) in atorvastatin and 12.1 (95% CI -2.5 to +27) in placebo subjects, a 10% non-significant reduction. This effect was greater in males (33% reduction) and in CCMs at brainstem location (76% reduction), both non-significant. There were no significant differences in SH rates, subclinical bleeds, vascular permeability of lesion or brain on dynamic contrast enhanced quantititve perfusion, or leukocyte Rho kinase activity. Cholesterol levels were lower and adverse events were more common with atorvastatin.
Interpretation. Atorvastatin treatment was safe in CCMs with recent SH, with a non-significantly decreased hemorrhage risk during 2 years of follow-up.
Funding. U.S. National Institutes of Health R01NS107887. Clinicaltrials.gov NCT02603328.
More abstracts on this topic:
Greenberg Steven, Parikh Neal, Lee Jin-moo, Van Etten Ellis, Van Osch Matthias, Klijn Catharina, Sostelly Alexandre, Goteti Sasikiran, Sepehrband Farshid, Avbersek Andreja, Deering Robert
Aging-Associate Peptide Medin Induces Proinflammatory Activation in Human Brain Vascular Smooth Muscle CellsKaramanova Nina, Morrow Kaleb, Maerivoet Alana, Madine Jillian, Lozoya Maria, Weissig Volkmar, Li Ming, Migrino Raymond
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.