Final ID: Sa4009
The Role and Mechanism of Receptor-like Protein LRTM1 in the Negative Regulation of Cardiomyocyte Regeneration
Abstract Body (Do not enter title and authors here): Background:
One of the core pathological features of myocardial infarction is the loss of cardiomyocytes, and promoting cardiomyocyte regeneration represents a potential therapeutic strategy for post-infarction heart failure.The aim of this research is to uncover the role and mechanisms of Receptor-like Protein LRTM1 in cardiomyocyte regeneration, providing novel therapeutic targets for cardiac repair.
Method:
In this study, we performed two cardiac injury models - apical resection and myocardial infarction operation - in both neonatal and adult mice. Cardiomyocyte-specific Lrtm1 deletion was achieved through combinatorial use of Myh6-MerCreMer and Myh6-iCre transgenic mouse lines with Lrtm1fl/fl conditional alleles. A series of molecular signaling experiments, including RNA sequencing, immunostaining and coimmunoprecipitation , were conducted. Heart regeneration and cardiac function were evaluated by Masson staining and echocardiography, respectively.
Result:
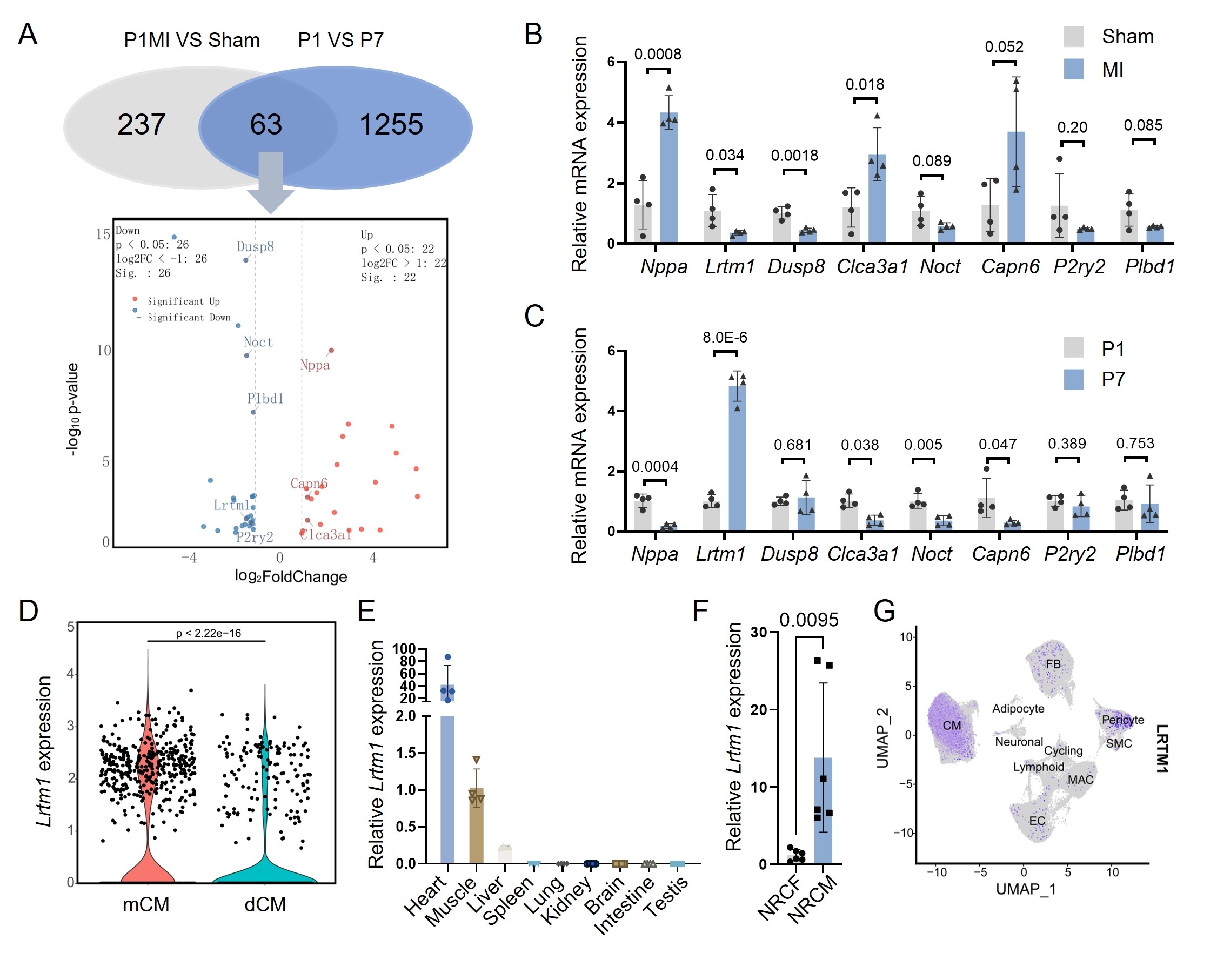
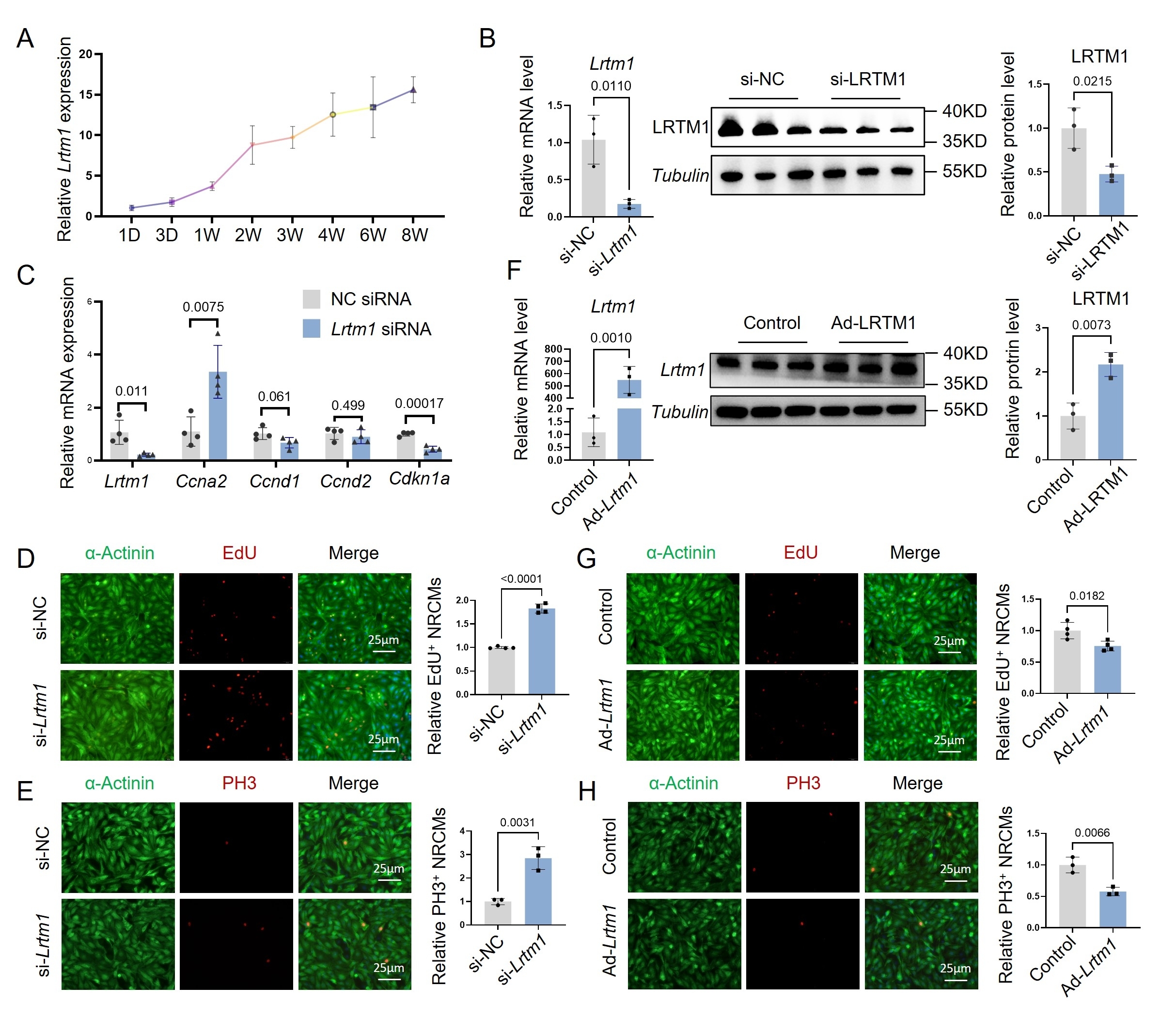
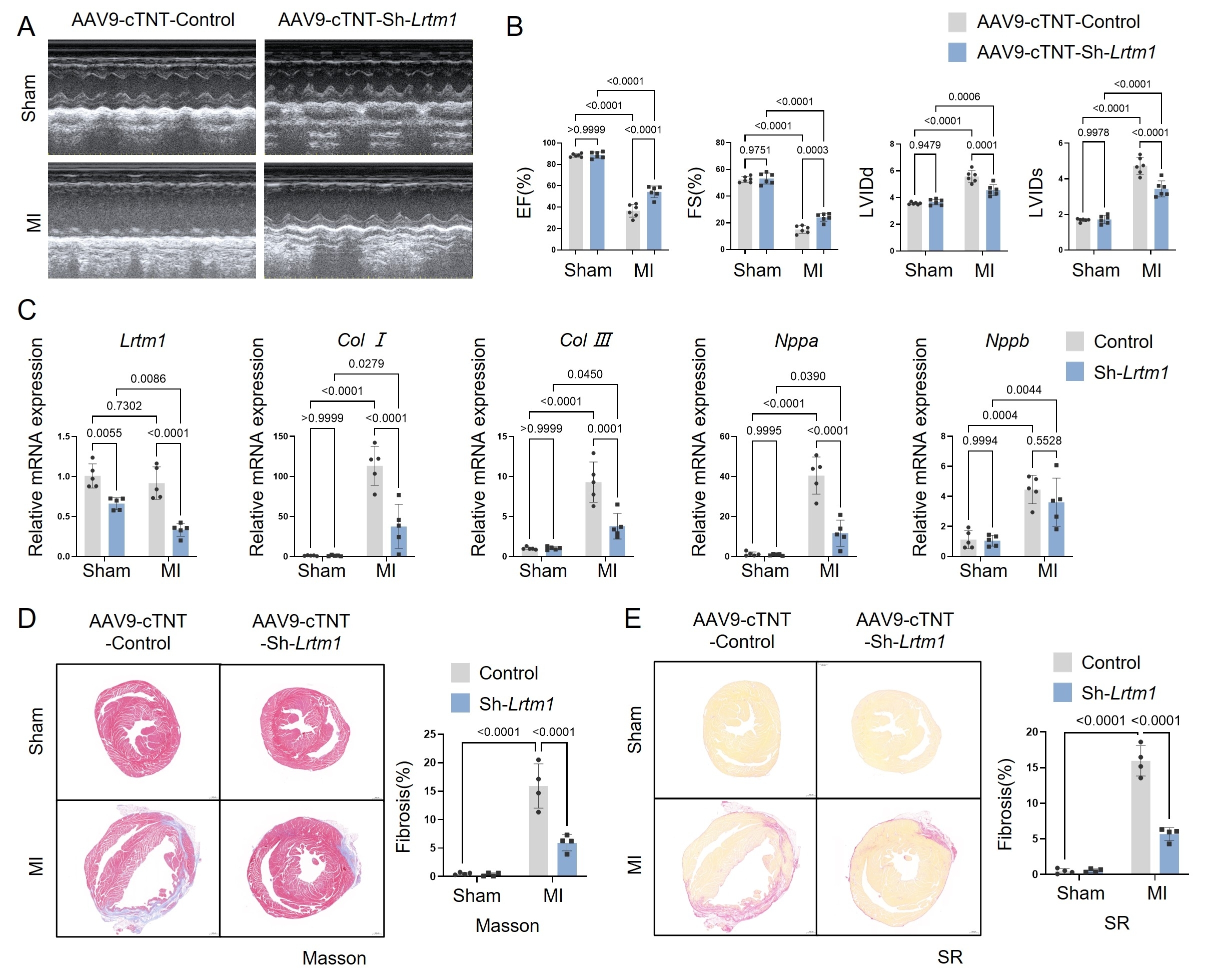
Integrated analysis of RNA-seq data from P1 and P7 murine hearts, as well as P1 myocardial infarction (MI) versus sham-operated hearts, revealed a negative correlation between LRTM1 expression and cardiomyocyte proliferative capacity. Notably, LRTM1 exhibited cardiac-specific enrichment, particularly in cardiomyocytes. These findings implicate LRTM1 as a critical regulator of intrinsic myocardial regeneration. In vitro and in vivo functional studies demonstrated that LRTM1 knockdown enhanced proliferation in neonatal rat primary cardiomyocytes, extended the postnatal cardiomyocyte proliferative temporal window in neonatal mice, and promoted cardiomyocyte proliferation in adult MI mice, thereby improving post-MI cardiac function and attenuating pathological ventricular remodeling. Mechanistically, combined RNA-seq and motif enrichment analyses revealed that LRTM1 downregulation suppressed JAK/STAT1 signaling activation by impairing extracellular IFN-α/receptor binding, leading to reduced IRF1 transcription factor expression. This cascade ultimately drives cardiomyocyte proliferation through coordinated modulation of cell cycle-related proteins.
Conclusion:
Cardiomyocyte-specific knockout of LRTM1 promotes cardiomyocyte proliferation, extends the proliferative time window in neonatal mice and ameliorates post-infarction cardiac remodeling in adult mice via interferon signaling.These findings support a potentially important new therapeutic approach for human heart failure
One of the core pathological features of myocardial infarction is the loss of cardiomyocytes, and promoting cardiomyocyte regeneration represents a potential therapeutic strategy for post-infarction heart failure.The aim of this research is to uncover the role and mechanisms of Receptor-like Protein LRTM1 in cardiomyocyte regeneration, providing novel therapeutic targets for cardiac repair.
Method:
In this study, we performed two cardiac injury models - apical resection and myocardial infarction operation - in both neonatal and adult mice. Cardiomyocyte-specific Lrtm1 deletion was achieved through combinatorial use of Myh6-MerCreMer and Myh6-iCre transgenic mouse lines with Lrtm1fl/fl conditional alleles. A series of molecular signaling experiments, including RNA sequencing, immunostaining and coimmunoprecipitation , were conducted. Heart regeneration and cardiac function were evaluated by Masson staining and echocardiography, respectively.
Result:
Integrated analysis of RNA-seq data from P1 and P7 murine hearts, as well as P1 myocardial infarction (MI) versus sham-operated hearts, revealed a negative correlation between LRTM1 expression and cardiomyocyte proliferative capacity. Notably, LRTM1 exhibited cardiac-specific enrichment, particularly in cardiomyocytes. These findings implicate LRTM1 as a critical regulator of intrinsic myocardial regeneration. In vitro and in vivo functional studies demonstrated that LRTM1 knockdown enhanced proliferation in neonatal rat primary cardiomyocytes, extended the postnatal cardiomyocyte proliferative temporal window in neonatal mice, and promoted cardiomyocyte proliferation in adult MI mice, thereby improving post-MI cardiac function and attenuating pathological ventricular remodeling. Mechanistically, combined RNA-seq and motif enrichment analyses revealed that LRTM1 downregulation suppressed JAK/STAT1 signaling activation by impairing extracellular IFN-α/receptor binding, leading to reduced IRF1 transcription factor expression. This cascade ultimately drives cardiomyocyte proliferation through coordinated modulation of cell cycle-related proteins.
Conclusion:
Cardiomyocyte-specific knockout of LRTM1 promotes cardiomyocyte proliferation, extends the proliferative time window in neonatal mice and ameliorates post-infarction cardiac remodeling in adult mice via interferon signaling.These findings support a potentially important new therapeutic approach for human heart failure
More abstracts on this topic:
Apical resection preserves the proliferative capacity of cardiomyocytes located throughout the left ventricle of the heart
Nguyen Thanh, Nakada Yuji, Wei Yuhua, Zhang Jianyi
Cardiac protection of hiPSC-CMs loaded chitosan cardiac patch in myocardial infarcted swineZheng Zilong, Li Yichen, Tang Weijie, Chen Wangping, Yang Jinfu, Fan Chengming